Abstract
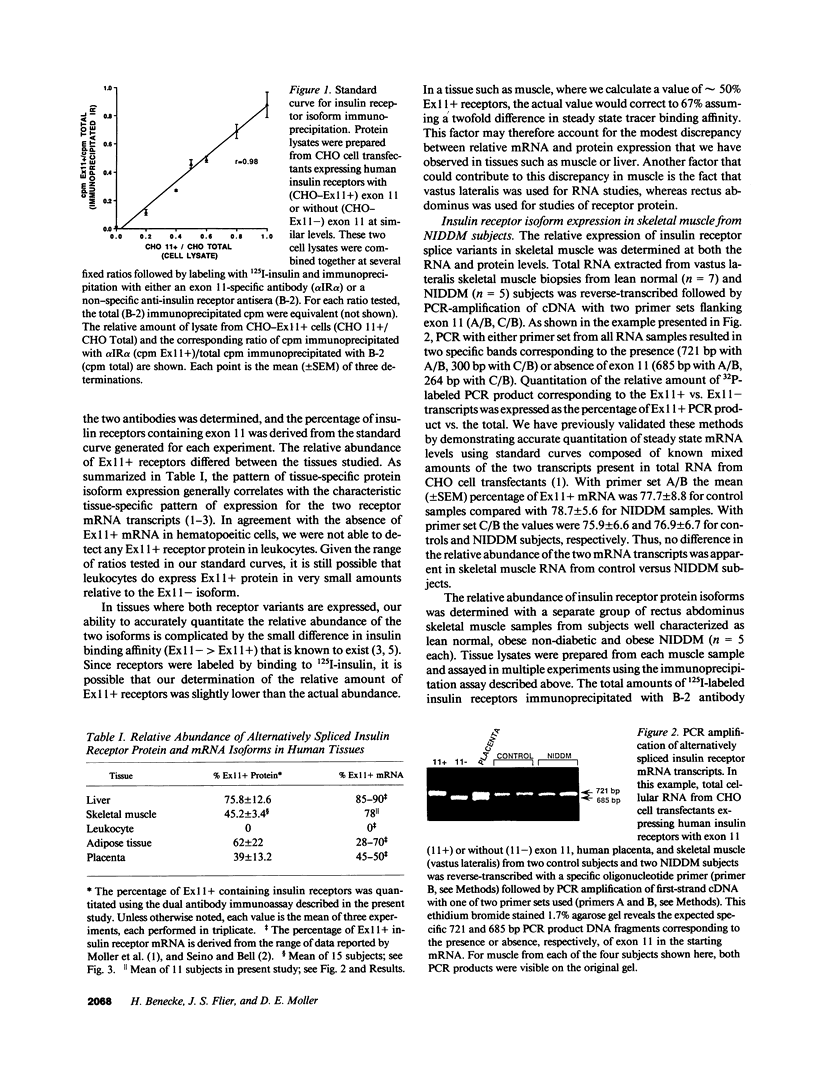
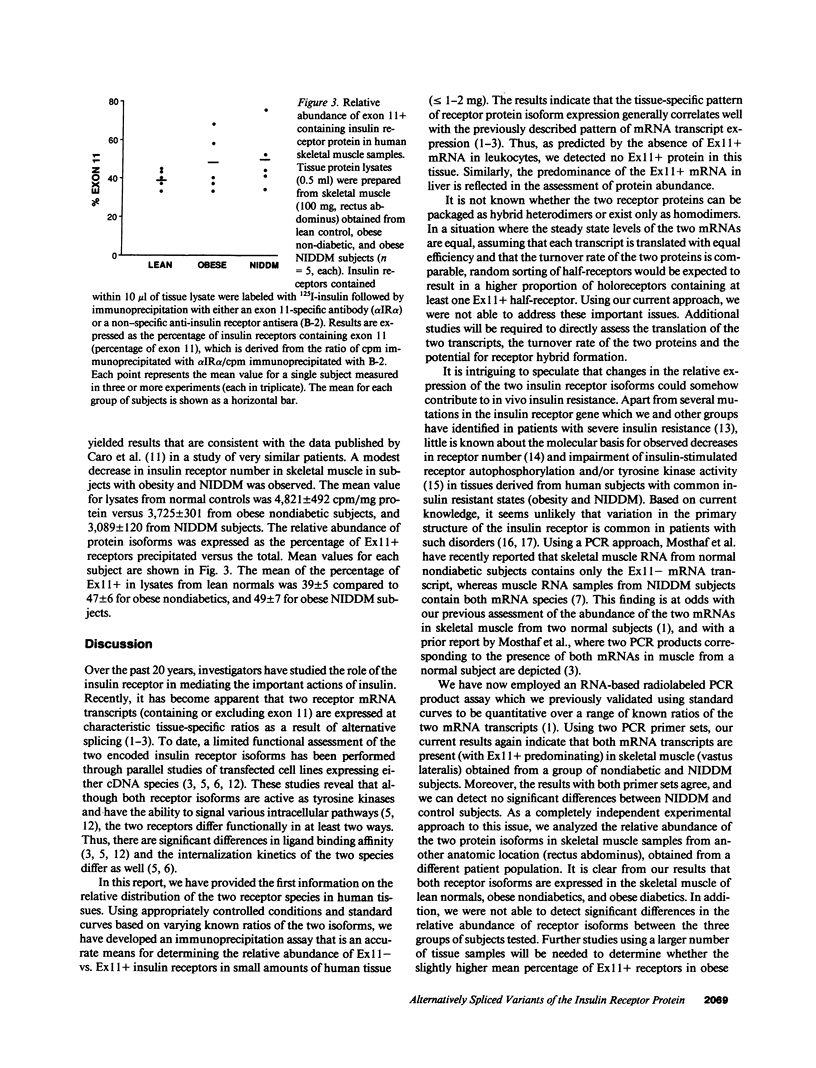
Two insulin receptor mRNA transcripts resulting from alternative splicing of exon 11 in the receptor gene are expressed in a highly regulated tissue-specific fashion. To date, there is no information about the relative abundance of the protein isoforms encoded by these mRNAs in tissues of normal or diabetic subjects. We employed an antibody raised against the peptide sequence encoded by exon 11 to develop a specific immunoprecipitation assay that is capable of determining the fraction of receptors that include this amino acid sequence. The assay is based on the relative ability of the exon 11 specific monoclonal antibody (alpha IR alpha) compared to a nonspecific anti-receptor antiserum (B-2) to immunoprecipitate solubilized receptors that are first labeled with 125I-insulin. The assay was validated using standard curves generated with samples composed of known ratios of the two receptor isoforms. Our results in general confirm observations regarding the relative abundance of the two mRNA species in human tissues, with marked predominance of the exon 11+ isoform in liver, and the exon 11- isoform in leukocytes. Similar amounts of both variants are present in placenta, skeletal muscle, and adipose tissue. In studies with this assay using skeletal muscle extracts from control and noninsulin-dependent diabetes mellitus (NIDDM) subjects, as well as in studies of the two mRNAs in control versus NIDDM muscle using a quantitative polymerase chain reaction assay, we could find no significant difference between control and diabetic subjects. This data contradicts a recent report claiming that normal individuals have only the exon 11- mRNA transcript in their skeletal muscle, whereas NIDDM subjects have similar expression of both mRNAs. Given the emerging evidence that functional differences exist between the two receptor isoforms, these studies are relevant to our understanding of insulin receptor function in health and disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest. 1976 Dec;58(6):1442–1449. doi: 10.1172/JCI108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. J., Dudley A. L. The rat insulin receptor: primary structure and conservation of tissue-specific alternative messenger RNA splicing. Mol Endocrinol. 1990 Feb;4(2):235–244. doi: 10.1210/mend-4-2-235. [DOI] [PubMed] [Google Scholar]
- Häring H., Obermaier-Kusser B. Insulin receptor kinase defects in insulin-resistant tissues and their role in the pathogenesis of NIDDM. Diabetes Metab Rev. 1989 Aug;5(5):431–441. doi: 10.1002/dmr.5610050502. [DOI] [PubMed] [Google Scholar]
- Kusari J., Verma U. S., Buse J. B., Henry R. R., Olefsky J. M. Analysis of the gene sequences of the insulin receptor and the insulin-sensitive glucose transporter (GLUT-4) in patients with common-type non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Oct;88(4):1323–1330. doi: 10.1172/JCI115437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A. Different ligand affinities of the two human insulin receptor splice variants are reflected in parallel changes in sensitivity for insulin action. Mol Endocrinol. 1991 May;5(5):734–739. doi: 10.1210/mend-5-5-734. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991 Sep 26;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Yokota A., Caro J. F., Flier J. S. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989 Aug;3(8):1263–1269. doi: 10.1210/mend-3-8-1263. [DOI] [PubMed] [Google Scholar]
- Mosthaf L., Grako K., Dull T. J., Coussens L., Ullrich A., McClain D. A. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990 Aug;9(8):2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosthaf L., Vogt B., Häring H. U., Ullrich A. Altered expression of insulin receptor types A and B in the skeletal muscle of non-insulin-dependent diabetes mellitus patients. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4728–4730. doi: 10.1073/pnas.88.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly S., Choi W. H., Patel P., Turner R. C., Flier J. S., Moller D. E. Detection of mutations in insulin-receptor gene in NIDDM patients by analysis of single-stranded conformation polymorphisms. Diabetes. 1991 Jun;40(6):777–782. doi: 10.2337/diab.40.6.777. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Seino S., Bell G. I. Alternative splicing of human insulin receptor messenger RNA. Biochem Biophys Res Commun. 1989 Feb 28;159(1):312–316. doi: 10.1016/0006-291x(89)92439-x. [DOI] [PubMed] [Google Scholar]
- Vogt B., Carrascosa J. M., Ermel B., Ullrich A., Häring H. U. The two isotypes of the human insulin receptor (HIR-A and HIR-B) follow different internalization kinetics. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1013–1018. doi: 10.1016/0006-291x(91)90639-o. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Flier J. S., Yokota A., Benecke H., Backer J. M., Moller D. E. Functional properties of two naturally occurring isoforms of the human insulin receptor in Chinese hamster ovary cells. Endocrinology. 1991 Oct;129(4):2058–2066. doi: 10.1210/endo-129-4-2058. [DOI] [PubMed] [Google Scholar]



