Abstract
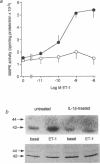
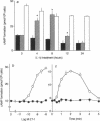
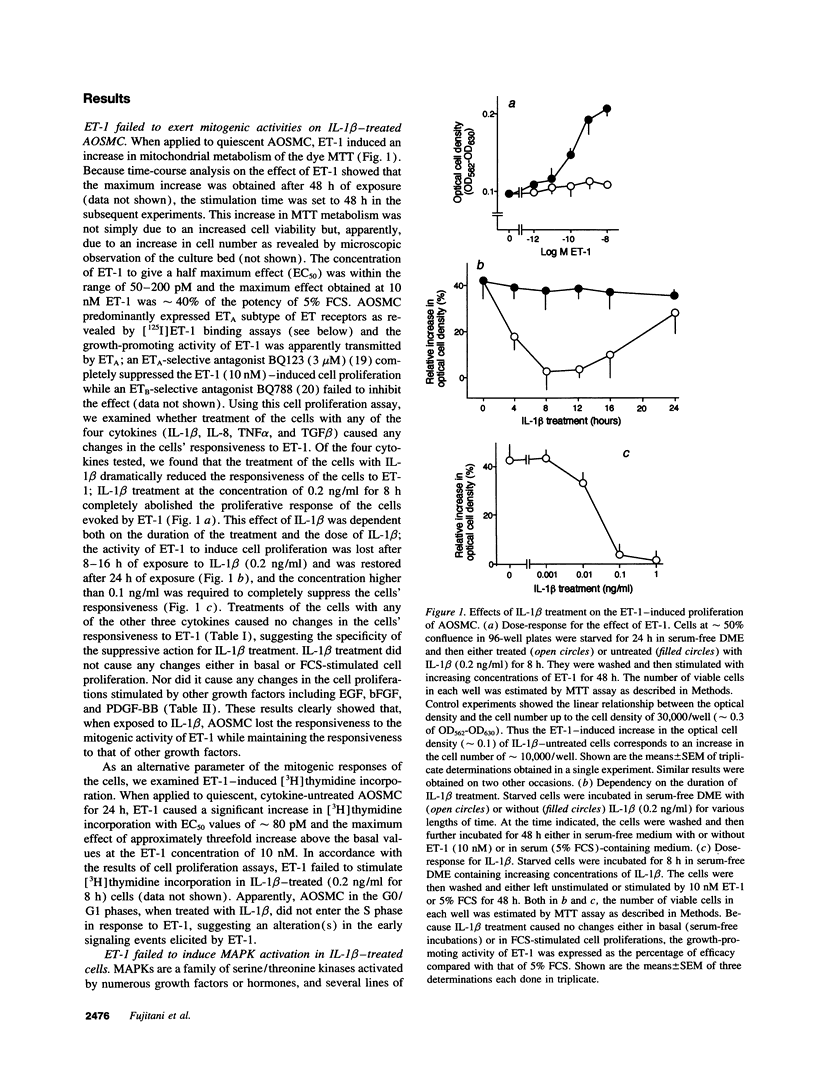
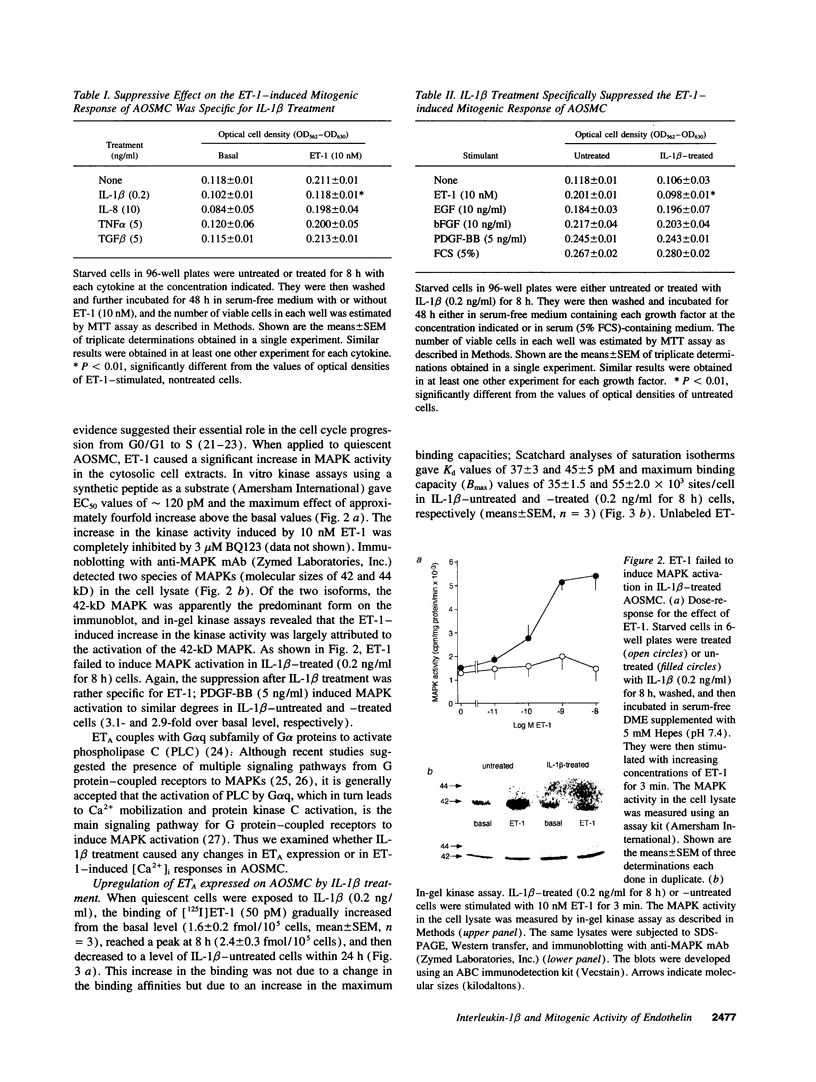
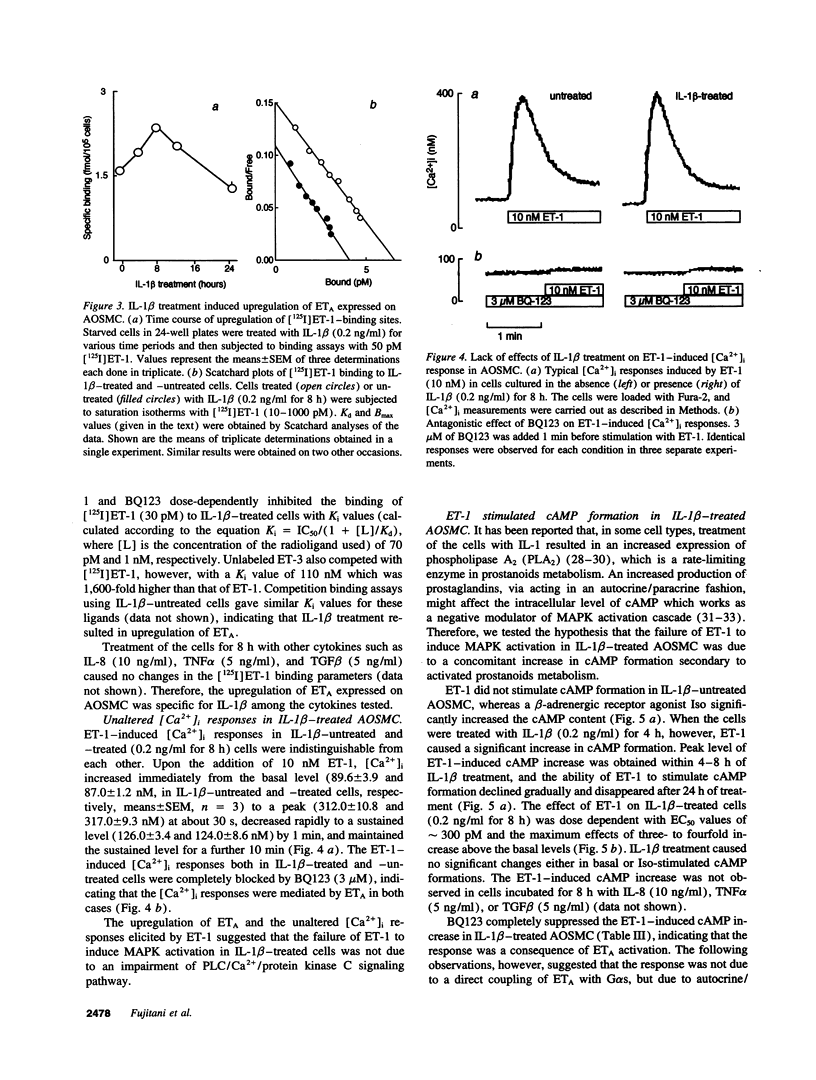
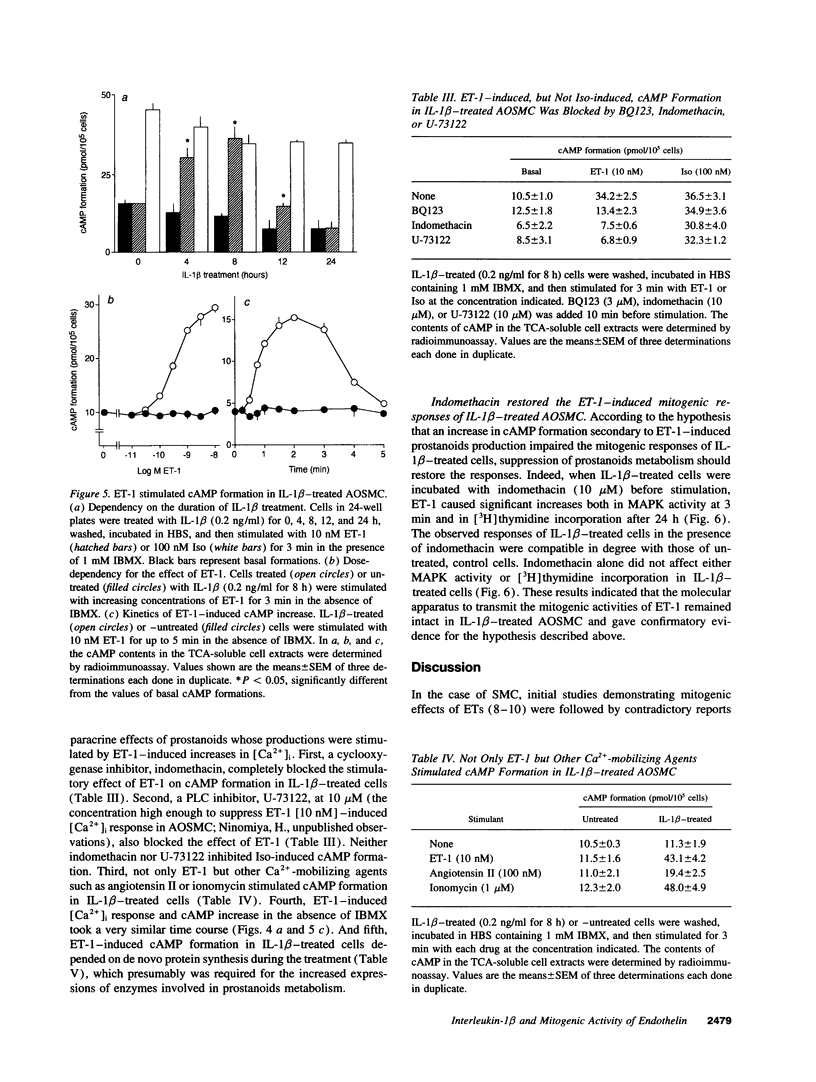
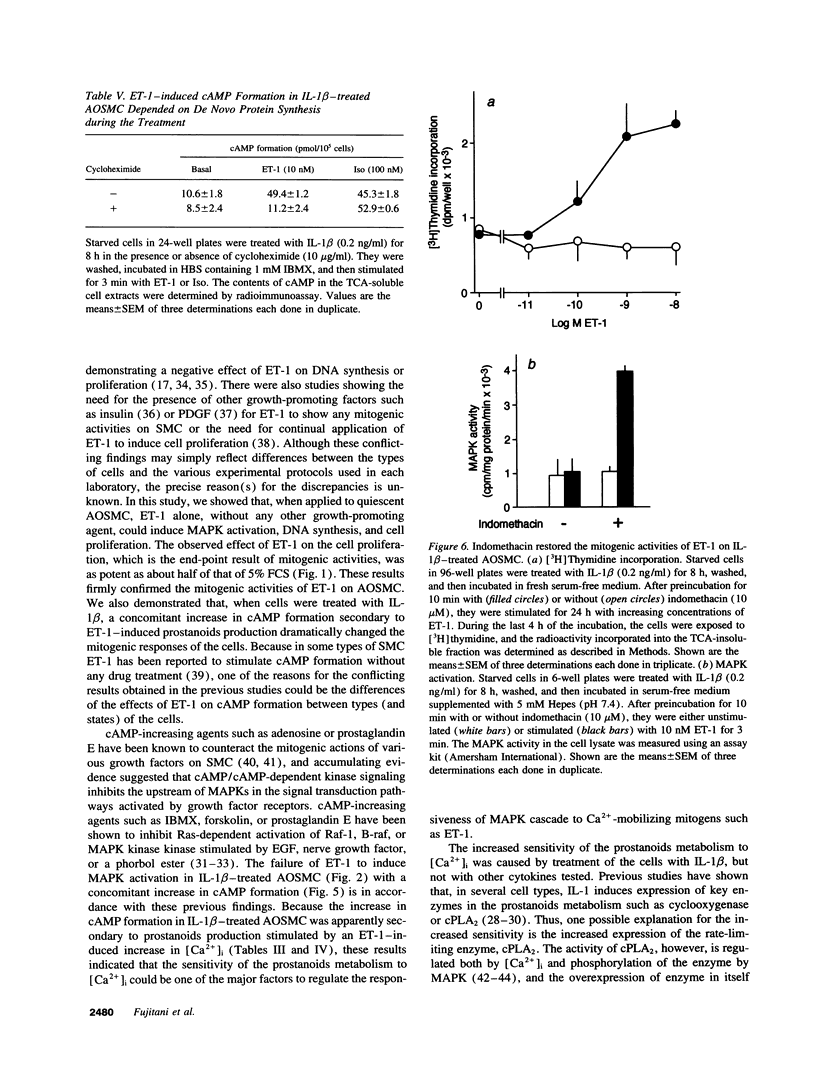
When applied to quiescent human aortic smooth muscle cells (AOSMC), endothelin-1 (ET-1) caused significant increases in mitogen-activated protein kinase (MAPK) activity, [3H]thymidine incorporation, and cell proliferation, confirming an activity of ET-1 as a potent mitogen on AOSMC. As an in vitro model to evaluate the significance of the mitogenic activity of ET-1 on smooth muscle cells during atherogenesis, we studied possible modulations of the responsiveness of the cells by treatment with various cytokines (IL-1 beta, IL-8, TNF alpha, and TGF beta). Of the four cytokines tested, we found that the treatment of the cells with IL-1 beta dramatically reduced the responsiveness of the cells to ET-1; IL-1 beta treatment at the concentration of 0.2 ng/ml for 8 h completely abolished the activity of ET-1 to induce the mitogenic responses. IL-1 beta treatment caused no changes in the responses induced by EGF, basic fibroblast growth factor, or PDGF. Studies on ET-1-induced intracellular signaling events in IL-1 beta-treated cells revealed that the failure of ET-1 to induce mitogenic responses was due to an increase in cAMP formation secondary to ET-1-induced activation of prostanoid metabolism. These findings on AOSMC in vitro raise the possibility that, under some inflammatory conditions in vivo, ETs may work as a negative modulator of smooth muscle cell proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts G. F., Peifley K. A., Johns A., Kleha J. F., Winkles J. A. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J Biol Chem. 1994 Apr 1;269(13):10112–10118. [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bobik A., Grooms A., Millar J. A., Mitchell A., Grinpukel S. Growth factor activity of endothelin on vascular smooth muscle. Am J Physiol. 1990 Mar;258(3 Pt 1):C408–C415. doi: 10.1152/ajpcell.1990.258.3.C408. [DOI] [PubMed] [Google Scholar]
- Boulanger C. M., Tanner F. C., Béa M. L., Hahn A. W., Werner A., Lüscher T. F. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992 Jun;70(6):1191–1197. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- Chua B. H., Krebs C. J., Chua C. C., Diglio C. A. Endothelin stimulates protein synthesis in smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):E412–E416. doi: 10.1152/ajpendo.1992.262.4.E412. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Crespo P., Xu N., Simonds W. F., Gutkind J. S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994 Jun 2;369(6479):418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Hirata Y., Imai T., Marumo F. Endothelin receptor subtypes are coupled to adenylate cyclase via different guanyl nucleotide-binding proteins in vasculature. Endocrinology. 1993 Feb;132(2):524–529. doi: 10.1210/endo.132.2.7678793. [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Oda K., Takimoto M., Inui T., Okada T., Urade Y. Autocrine receptors for endothelins in the primary culture of endothelial cells of human umbilical vein. FEBS Lett. 1992 Feb 17;298(1):79–83. doi: 10.1016/0014-5793(92)80026-d. [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Ueda H., Okada T., Urade Y., Karaki H. A selective agonist of endothelin type B receptor, IRL 1620, stimulates cyclic GMP increase via nitric oxide formation in rat aorta. J Pharmacol Exp Ther. 1993 Nov;267(2):683–689. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirata Y., Takagi Y., Fukuda Y., Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989 Aug;78(2-3):225–228. doi: 10.1016/0021-9150(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Hosoda K., Nakao K., Hiroshi-Arai, Suga S., Ogawa Y., Mukoyama M., Shirakami G., Saito Y., Nakanishi S., Imura H. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett. 1991 Aug 5;287(1-2):23–26. doi: 10.1016/0014-5793(91)80007-p. [DOI] [PubMed] [Google Scholar]
- Ihara M., Noguchi K., Saeki T., Fukuroda T., Tsuchida S., Kimura S., Fukami T., Ishikawa K., Nishikibe M., Yano M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50(4):247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Ihara M., Noguchi K., Mase T., Mino N., Saeki T., Fukuroda T., Fukami T., Ozaki S., Nagase T. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. F., Xie L. H., Fujitani Y., Hayashi S., Horie M. Inhibition of the cardiac protein kinase A-dependent chloride conductance by endothelin-1. Nature. 1994 Jul 28;370(6487):297–300. doi: 10.1038/370297a0. [DOI] [PubMed] [Google Scholar]
- Jonzon B., Nilsson J., Fredholm B. B. Adenosine receptor-mediated changes in cyclic AMP production and DNA synthesis in cultured arterial smooth muscle cells. J Cell Physiol. 1985 Sep;124(3):451–456. doi: 10.1002/jcp.1041240314. [DOI] [PubMed] [Google Scholar]
- Kerr J. S., Stevens T. M., Davis G. L., McLaughlin J. A., Harris R. R. Effects of recombinant interleukin-1 beta on phospholipase A2 activity, phospholipase A2 mRNA levels, and eicosanoid formation in rabbit chondrocytes. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1079–1084. doi: 10.1016/0006-291x(89)92712-5. [DOI] [PubMed] [Google Scholar]
- Koide M., Kawahara Y., Tsuda T., Ishida Y., Shii K., Yokoyama M. Endothelin-1 stimulates tyrosine phosphorylation and the activities of two mitogen-activated protein kinases in cultured vascular smooth muscle cells. J Hypertens. 1992 Oct;10(10):1173–1182. doi: 10.1097/00004872-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Johnson G. L. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994 Sep 2;265(5177):1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lerman A., Edwards B. S., Hallett J. W., Heublein D. M., Sandberg S. M., Burnett J. C., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991 Oct 3;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., DeWitt D. L. Interleukin-1 alpha induces the accumulation of cytosolic phospholipase A2 and the release of prostaglandin E2 in human fibroblasts. J Biol Chem. 1992 Nov 25;267(33):23451–23454. [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Vande Woude G. F., Ahn N. G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994 Aug 12;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Martin-Nizard F., Houssaini H. S., Lestavel-Delattre S., Duriez P., Fruchart J. C. Modified low density lipoproteins activate human macrophages to secrete immunoreactive endothelin. FEBS Lett. 1991 Nov 18;293(1-2):127–130. doi: 10.1016/0014-5793(91)81167-7. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Sugishita Y., Matsuda M., Sakai H., Suzuki N., Masaki T., Goto K. Increased plasma concentration of endothelin-1 in cholesterol-fed rats. Atherosclerosis. 1992 Apr;93(3):257–259. doi: 10.1016/0021-9150(92)90263-g. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993 Jan 25;268(3):1960–1964. [PubMed] [Google Scholar]
- Nilsson J., Olsson A. G. Prostaglandin E1 inhibits DNA synthesis in arterial smooth muscle cells stimulated with platelet-derived growth factor. Atherosclerosis. 1984 Oct;53(1):77–82. doi: 10.1016/0021-9150(84)90107-2. [DOI] [PubMed] [Google Scholar]
- Ono K., Tsujimoto G., Sakamoto A., Eto K., Masaki T., Ozaki Y., Satake M. Endothelin-A receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994 Jul 28;370(6487):301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- Pernas P., Masliah J., Olivier J. L., Salvat C., Rybkine T., Bereziat G. Type II phospholipase A2 recombinant overexpression enhances stimulated arachidonic acid release. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1298–1305. doi: 10.1016/0006-291x(91)91035-b. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Leighton J., Pignat W., Märki F., Vosbeck K. Cyclic AMP mimics, but does not mediate, interleukin-1- and tumour-necrosis-factor-stimulated phospholipase A2 secretion from rat renal mesangial cells. Biochem J. 1991 Jan 1;273(Pt 1):199–204. doi: 10.1042/bj2730199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Vanhoutte P. M. Induction of endothelin secretion by angiotensin II: effects on growth and synthetic activity of vascular smooth muscle cells. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S96–100. [PubMed] [Google Scholar]
- Sun H., Tonks N. K., Bar-Sagi D. Inhibition of Ras-induced DNA synthesis by expression of the phosphatase MKP-1. Science. 1994 Oct 14;266(5183):285–288. doi: 10.1126/science.7939666. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Kasuya Y., Takuwa N., Kudo M., Yanagisawa M., Goto K., Masaki T., Yamashita K. Endothelin receptor is coupled to phospholipase C via a pertussis toxin-insensitive guanine nucleotide-binding regulatory protein in vascular smooth muscle cells. J Clin Invest. 1990 Mar;85(3):653–658. doi: 10.1172/JCI114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg P. L., Witchell C., Davenport A. P., Hesketh T. R., Metcalfe J. C. The endothelin peptides ET-1, ET-2, ET-3 and sarafotoxin S6b are co-mitogenic with platelet-derived growth factor for vascular smooth muscle cells. Atherosclerosis. 1990 Dec;85(2-3):257–262. doi: 10.1016/0021-9150(90)90118-3. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]