Abstract
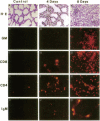
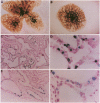
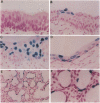
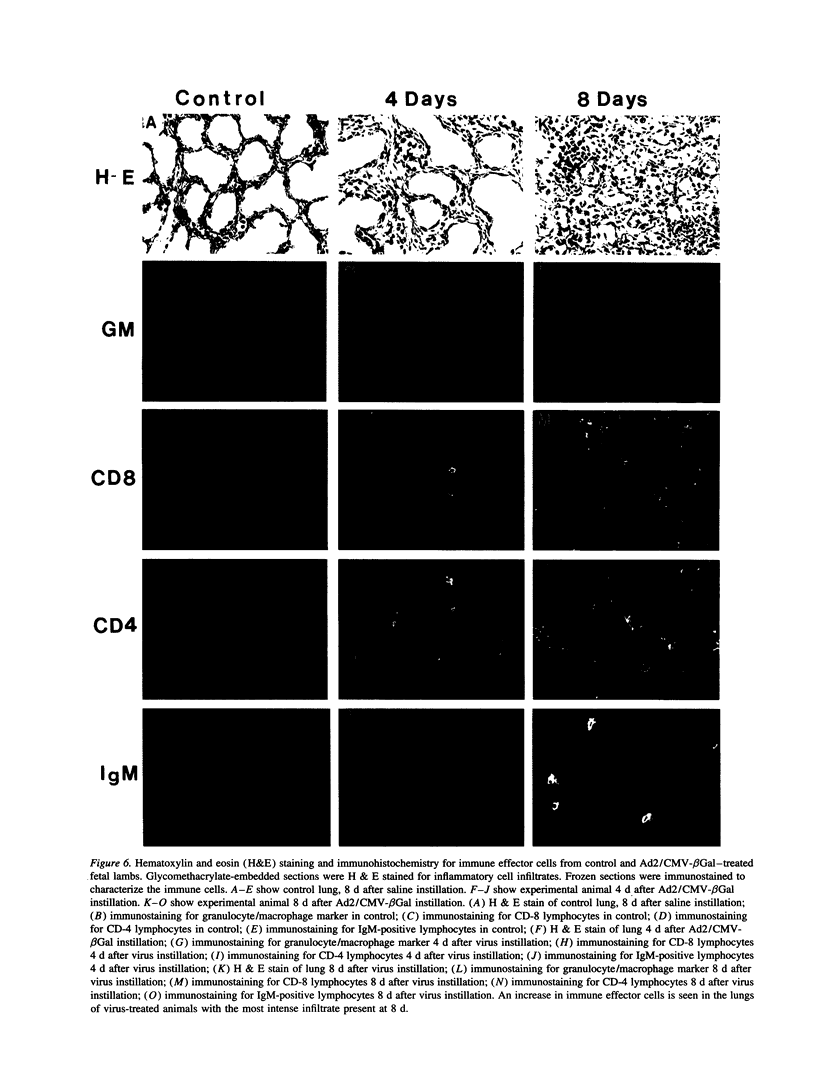
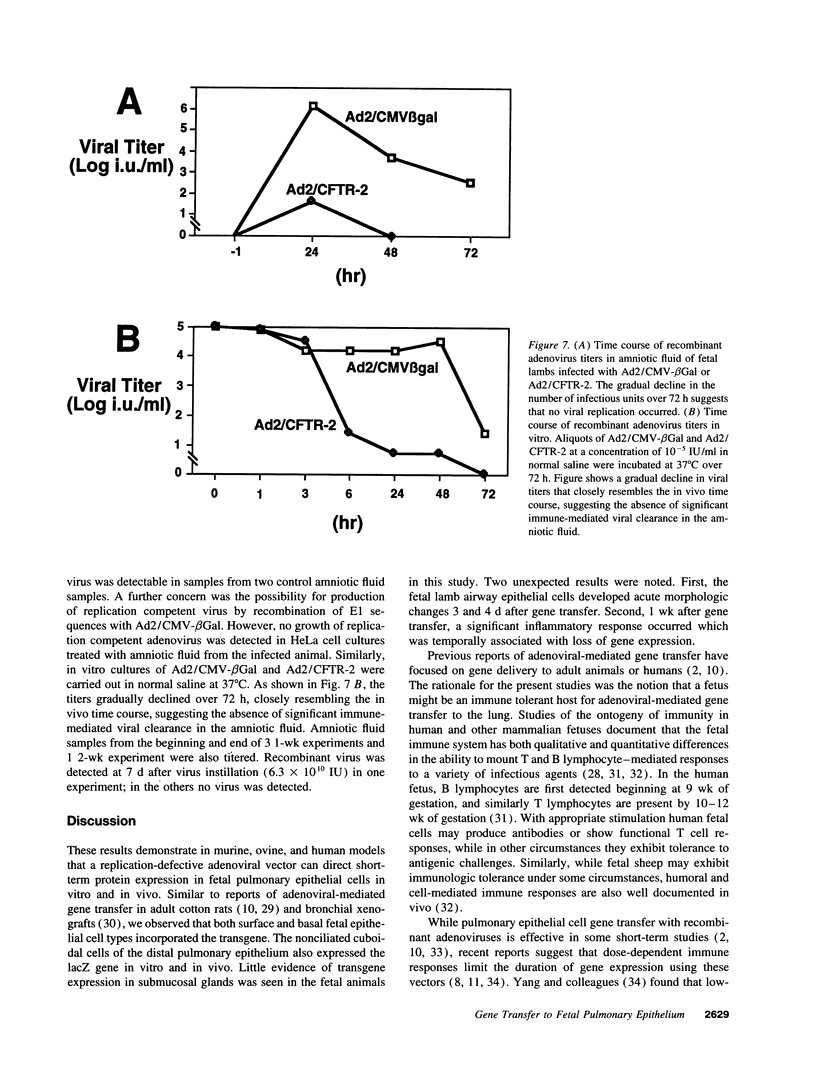
Vector-mediated gene transfer offers a direct method of correcting genetic pulmonary diseases and might also be used to correct temporary abnormalities associated with acquired, nongenetic disorders. Because the fetus or newborn may be a more immune tolerant host for gene transfer using viral vectors, we used replication defective recombinant adenoviral vectors to test the feasibility of gene transfer to the fetal pulmonary epithelium in vitro and in vivo. Both proximal and distal epithelial cells in cultured fetal lung tissues from rodents and humans diffusely expressed the lacZ transgene 3 d after viral infection. In vivo gene delivery experiments were performed in fetal mice and lambs. Delivery of Ad2/CMV-beta Gal to the amniotic fluid in mice produced intense transgene expression in the fetal epidermis and amniotic membranes, some gastrointestinal expression, but no significant airway epithelial expression. When we introduced the adenoviral vector directly into the trachea of fetal lambs, the lacZ gene was expressed in the tracheal, bronchial, and distal pulmonary epithelial cells 3 d after viral infection. Unexpectedly, reactive hyperplasia and squamous metaplasia were noted in epithelia expressing lacZ in the trachea, but not in the distal lung of fetal lambs. 1 wk after infection, adenovirus-treated fetuses developed inflammatory cell infiltrates in the lung tissue with CD4, CD8, IgM, and granulocyte/macrophage positive immune effector cells. Transgene expression faded coincident with inflammation and serologic evidence of antiadenoviral antibody production. While these studies document the feasibility of viral-mediated gene transfer in the prenatal lung, they indicate that immunologic responses to E1-deleted recombinant adenoviruses limit the duration of transgene expression.
Full text
PDF


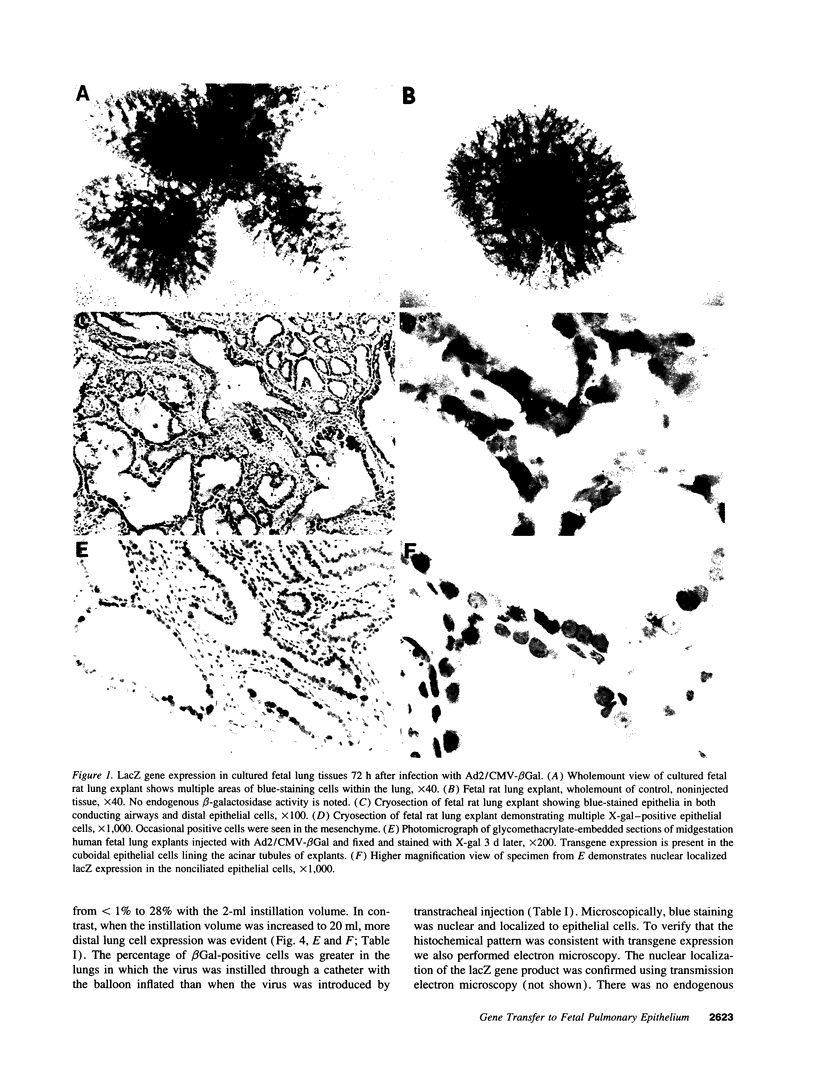
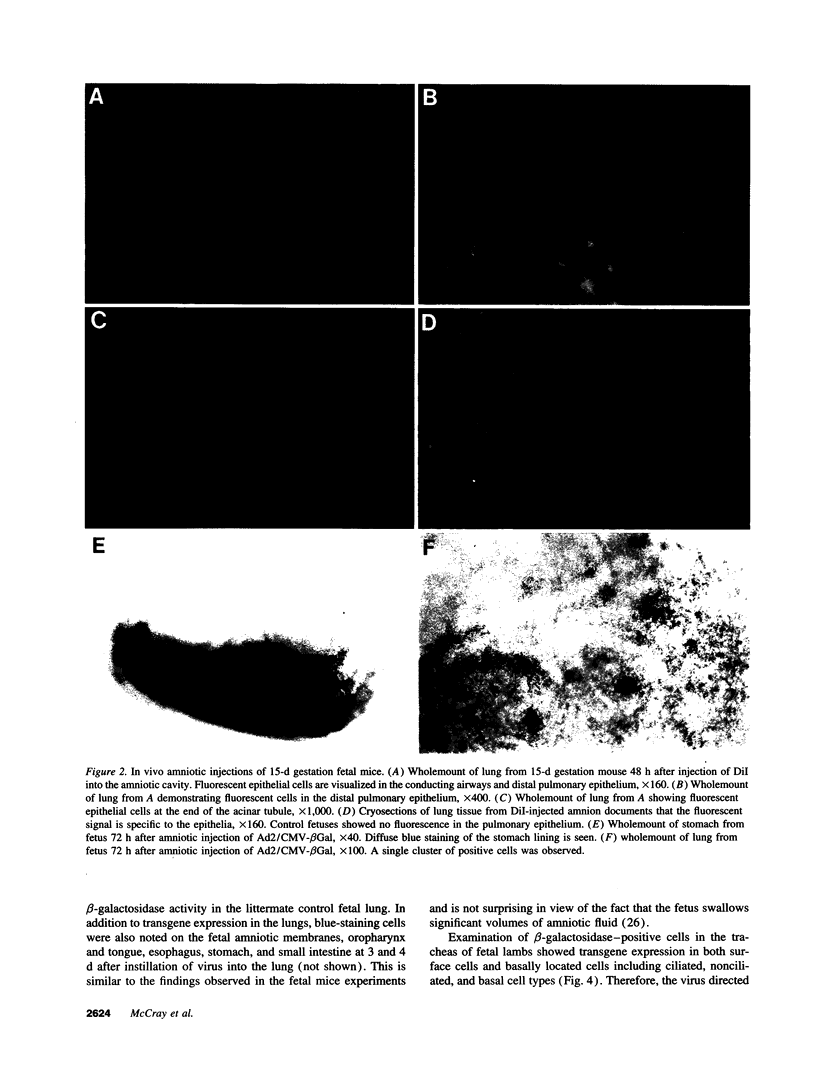
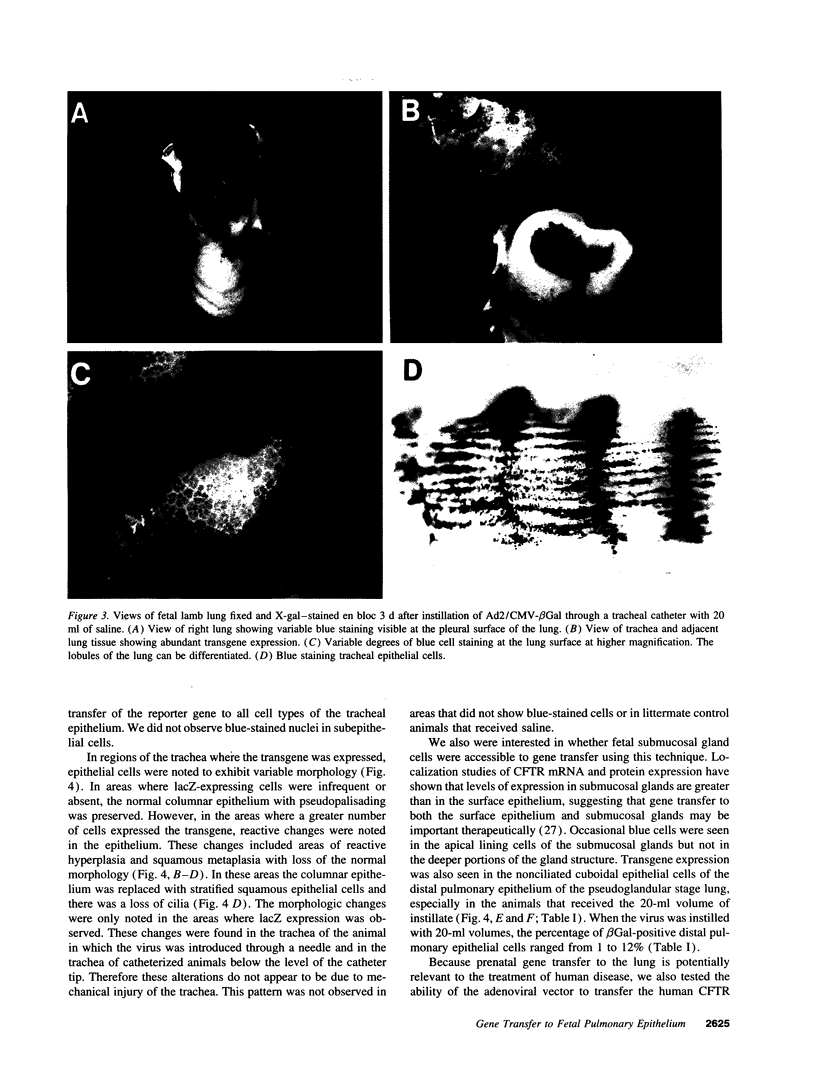

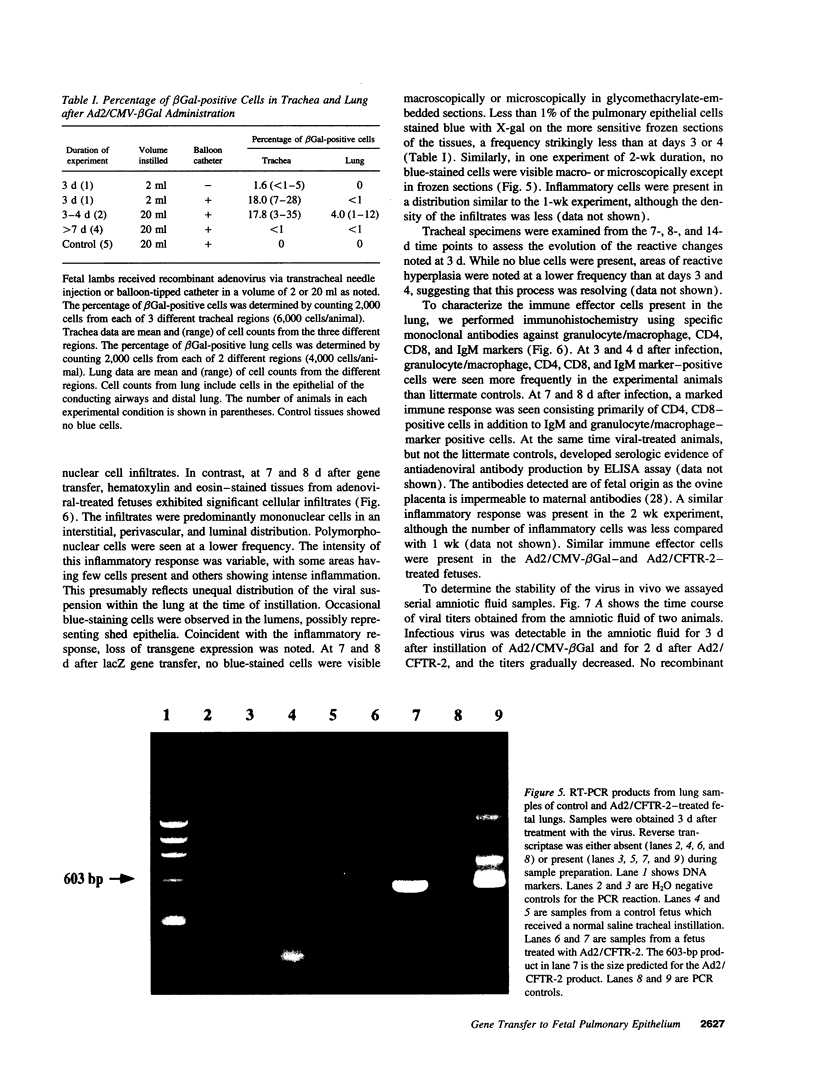



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artinger K. B., Bronner-Fraser M. Partial restriction in the developmental potential of late emigrating avian neural crest cells. Dev Biol. 1992 Jan;149(1):149–157. doi: 10.1016/0012-1606(92)90271-h. [DOI] [PubMed] [Google Scholar]
- Becker S., Koren H. S., Henke D. C. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1, and interleukin-6. Am J Respir Cell Mol Biol. 1993 Jan;8(1):20–27. doi: 10.1165/ajrcmb/8.1.20. [DOI] [PubMed] [Google Scholar]
- Campbell S. G., Siegel M. J., Knowlton B. J. Sheep immunoglobulins and their transmission to the neonatal lamb. N Z Vet J. 1977 Dec;25(12):361–365. doi: 10.1080/00480169.1977.34458. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Rossier B. C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993 Feb 4;361(6411):467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis W. C., Marusic S., Lewin H. A., Splitter G. A., Perryman L. E., McGuire T. C., Gorham J. R. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet Immunol Immunopathol. 1987 Jul;15(4):337–376. doi: 10.1016/0165-2427(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Simon R. H., Yang Y., Zepeda M., Weber-Pendleton S., Doranz B., Grossman M., Wilson J. M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther. 1993 Dec;4(6):759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yang Y., Stratford-Perricaudet L. D., Allen E. D., Kozarsky K., Perricaudet M., Yankaskas J. R., Wilson J. M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993 May;4(1):27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yankaskas J. R., Ernst S. A., Yang Y., Marino C. R., Boucher R. C., Cohn J. A., Wilson J. M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992 Nov;2(3):240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yankaskas J. R., Wilson J. M. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992 Dec;90(6):2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen C. W., Jr, Lawson E. E., Gingras J., Boucher R. C., Gatzy J. T., Knowles M. R. Electrical potential difference and ion transport across nasal epithelium of term neonates: correlation with mode of delivery, transient tachypnea of the newborn, and respiratory rate. J Pediatr. 1988 Jul;113(1 Pt 1):121–127. doi: 10.1016/s0022-3476(88)80545-6. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Ross A., Dutia B. M. Summary of workshop findings of leukocyte antigens in sheep. Vet Immunol Immunopathol. 1993 Nov;39(1-3):49–59. doi: 10.1016/0165-2427(93)90163-x. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Massoud E. A., Sekhon H. S., Rotschild A., Thurlbeck W. M. The in vitro effect of triamcinolone acetonide on branching morphogenesis in the fetal rat lung. Pediatr Pulmonol. 1992 Sep;14(1):28–36. doi: 10.1002/ppul.1950140107. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A., Danel C., Rosenfeld M. A., Stratford-Perricaudet L., Perricaudet M., Pavirani A., Lecocq J. P., Crystal R. G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993 Jan;91(1):225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray P. B., Jr, Bettencourt J. D., Bastacky J., Denning G. M., Welsh M. J. Expression of CFTR and a cAMP-stimulated chloride secretory current in cultured human fetal alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993 Dec;9(6):578–585. doi: 10.1165/ajrcmb/9.6.578. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., Morris B. The ontogeny of the lymphoid system and immune responsiveness in sheep. Prog Vet Microbiol Immunol. 1988;4:21–55. [PubMed] [Google Scholar]
- Nogee L. M., Garnier G., Dietz H. C., Singer L., Murphy A. M., deMello D. E., Colten H. R. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994 Apr;93(4):1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogee L. M., de Mello D. E., Dehner L. P., Colten H. R. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993 Feb 11;328(6):406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Couture L. A., Cardoza L. M., Guiggio V. M., Armentano D., Espino P. C., Hehir K., Welsh M. J., Smith A. E., Gregory R. J. Development and analysis of recombinant adenoviruses for gene therapy of cystic fibrosis. Hum Gene Ther. 1993 Aug;4(4):461–476. doi: 10.1089/hum.1993.4.4-461. [DOI] [PubMed] [Google Scholar]
- Robillard J. E., Weitzman R. E. Developmental aspects of the fetal renal response to exogenous arginine vasopressin. Am J Physiol. 1980 May;238(5):F407–F414. doi: 10.1152/ajprenal.1980.238.5.F407. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Engelhardt J. F., Yang Y., Zepeda M., Weber-Pendleton S., Grossman M., Wilson J. M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993 Dec;4(6):771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- Snyder J. M., Johnston J. M., Mendelson C. R. Differentiation of type II cells of human fetal lung in vitro. Cell Tissue Res. 1981;220(1):17–25. doi: 10.1007/BF00209962. [DOI] [PubMed] [Google Scholar]
- Spergel J. M., Chen-Kiang S. Interleukin 6 enhances a cellular activity that functionally substitutes for E1A protein in transactivation. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6472–6476. doi: 10.1073/pnas.88.15.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel J. M., Hsu W., Akira S., Thimmappaya B., Kishimoto T., Chen-Kiang S. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J Virol. 1992 Feb;66(2):1021–1030. doi: 10.1128/jvi.66.2.1021-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang L. B. Fetal lung liquid: secretion and reabsorption. Physiol Rev. 1991 Oct;71(4):991–1016. doi: 10.1152/physrev.1991.71.4.991. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino C., Anderson R., Muneoka K. 3T3 cell integration and differentiative potential during limb development in the mouse. Dev Biol. 1993 Jan;155(1):38–45. doi: 10.1006/dbio.1993.1004. [DOI] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- Yang Y., Ertl H. C., Wilson J. M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994 Aug;1(5):433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raper S. E., Cohn J. A., Engelhardt J. F., Wilson J. M. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4601–4605. doi: 10.1073/pnas.90.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Couture L. A., Gregory R. J., Graham S. M., Smith A. E., Welsh M. J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993 Oct 22;75(2):207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- Zabner J., Petersen D. M., Puga A. P., Graham S. M., Couture L. A., Keyes L. D., Lukason M. J., St George J. A., Gregory R. J., Smith A. E. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994 Jan;6(1):75–83. doi: 10.1038/ng0194-75. [DOI] [PubMed] [Google Scholar]