Abstract
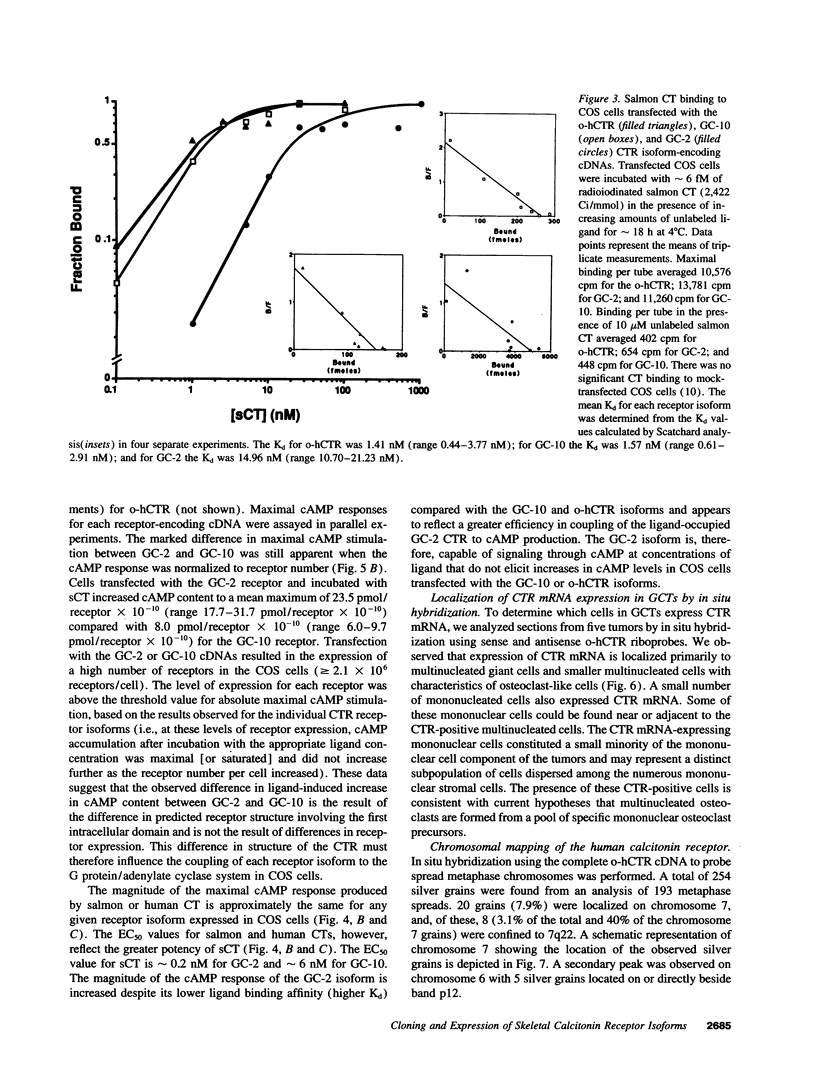
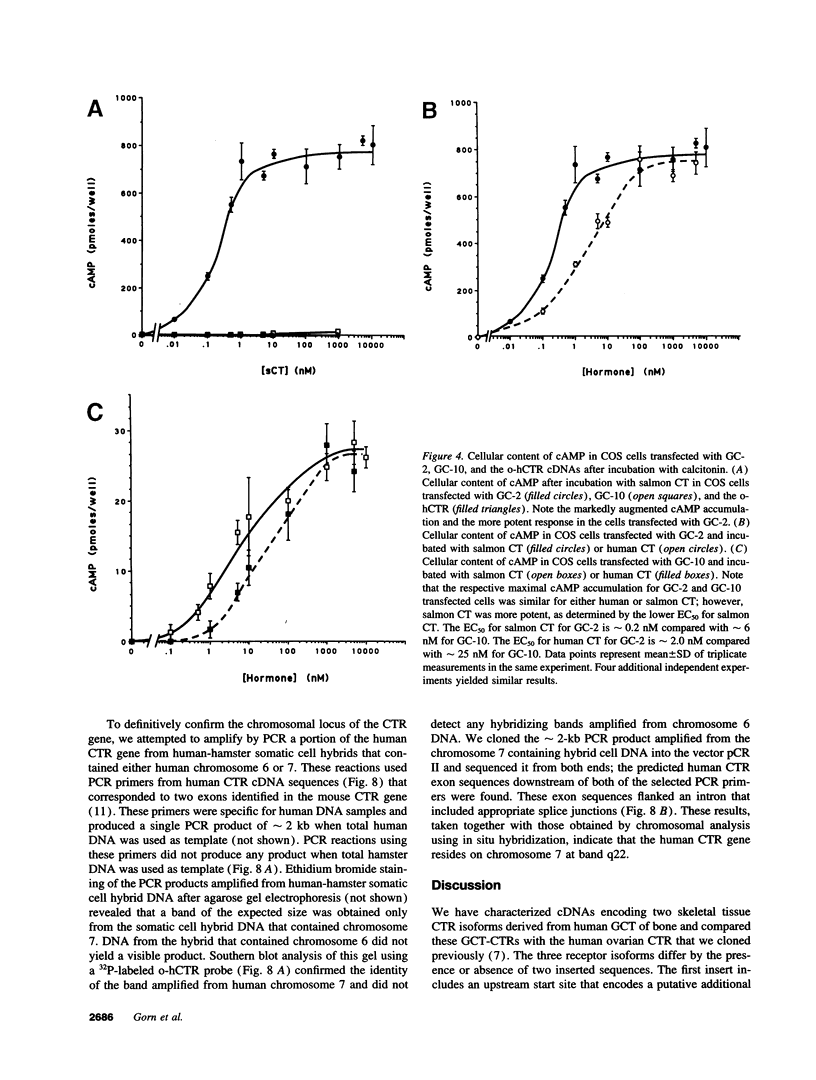
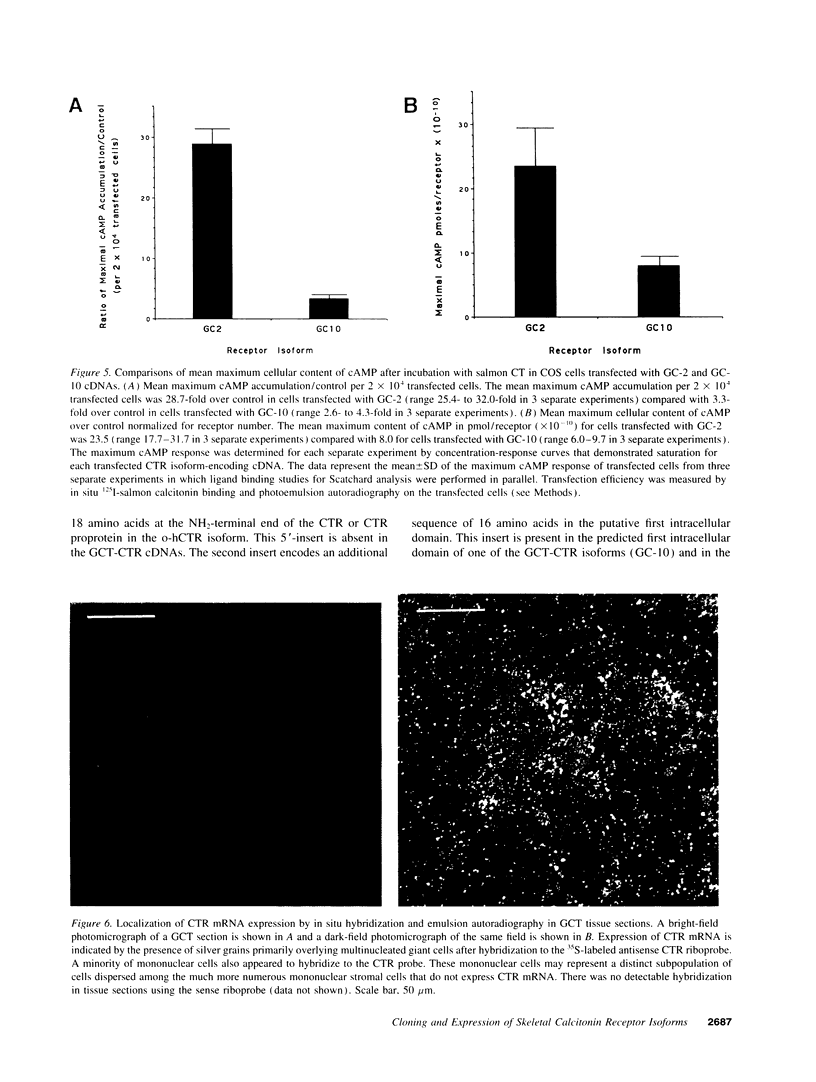
Two distinct calcitonin (CT) receptor (CTR)-encoding cDNAs (designated GC-2 and GC-10) were cloned and characterized from giant cell tumor of bone (GCT). Both GC-2 and GC-10 differ structurally from the human ovarian cell CTR (o-hCTR) that we cloned previously, but differ from each other only by the presence (GC-10) or absence (GC-2) of a predicted 16-amino acid insert in the putative first intracellular domain. Expression of all three CTR isoforms in COS cells demonstrated that GC-2 has a lower binding affinity for salmon (s) CT (Kd approximately 15 nM) than GC-10 or o-hCTR (Kd approximately 1.5 nM). Maximal stimulatory concentrations of CT resulted in a mean accumulation of cAMP in GC-2 transfected cells that was greater than eight times higher than in cells transfected with GC-10 after normalizing for the number of receptor-expressing cells. The marked difference in maximal cAMP response was also apparent after normalizing for receptor number. GC-2 also demonstrated a more potent ligand-mediated cAMP response compared with GC-10 for both human (h) and sCT (the EC50 values for GC-2 were approximately 0.2 nM for sCT and approximately 2 nM for hCT; EC50 values for GC-10 were approximately 6 nM for sCT and approximately 25 nM for hCT). Reverse transcriptase PCR of GCT RNA indicated that GC-2 transcripts are more abundant than those encoding for GC-10. In situ hybridization on GCT tissue sections demonstrated CTR mRNA expression in osteoclast-like cells. We localized the human CTR gene to chromosome 7 in band q22. The distinct functional characteristics of GC-2 and GC-10, which differ in structure only in the first intracellular domain, indicate that the first intracellular domain of the CTR plays a previously unidentified role in modulating ligand binding and signal transduction via the G protein/adenylate cyclase system.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrandt K., Mull E., Brady E. M., Herich J., Moore C. X., Beaumont K. Molecular cloning of two receptors from rat brain with high affinity for salmon calcitonin. FEBS Lett. 1993 Jul 5;325(3):225–232. doi: 10.1016/0014-5793(93)81078-e. [DOI] [PubMed] [Google Scholar]
- Byers V. S., Levin A. S., Johnston J. O., Hackett A. J. Quantitative immunofluorescence studies of the tumor antigen-bearing cell in giant cell tumor of bone and osteogenic sarcoma. Cancer Res. 1975 Sep;35(9):2520–2531. [PubMed] [Google Scholar]
- Chabre O., Conklin B. R., Lin H. Y., Lodish H. F., Wilson E., Ives H. E., Catanzariti L., Hemmings B. A., Bourne H. R. A recombinant calcitonin receptor independently stimulates 3',5'-cyclic adenosine monophosphate and Ca2+/inositol phosphate signaling pathways. Mol Endocrinol. 1992 Apr;6(4):551–556. doi: 10.1210/mend.6.4.1316547. [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Chatterjee D., Kellokumpu S., Rasmussen H., Baron R. Cell cycle-dependent coupling of the calcitonin receptor to different G proteins. Science. 1991 Mar 1;251(4997):1078–1082. doi: 10.1126/science.1847755. [DOI] [PubMed] [Google Scholar]
- Chausmer A., Stuart C., Stevens M. Identification of testicular cell plasma membrane receptors for calcitonin. J Lab Clin Med. 1980 Nov;96(5):933–938. [PubMed] [Google Scholar]
- Chen R., Lewis K. A., Perrin M. H., Vale W. W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. H., Huang R. R., Strader C. D. Involvement of specific hydrophobic, but not hydrophilic, amino acids in the third intracellular loop of the beta-adrenergic receptor in the activation of Gs. Mol Pharmacol. 1992 Jun;41(6):1061–1065. [PubMed] [Google Scholar]
- Claros M. G., von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994 Dec;10(6):685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Compton T., Ivanov I. E., Gottlieb T., Rindler M., Adesnik M., Sabatini D. D. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S., Exum S., Caron M. G., Lefkowitz R. J. Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2896–2900. doi: 10.1073/pnas.87.8.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman H. M., Neubig R. R. Two peptides from the alpha 2A-adrenergic receptor alter receptor G protein coupling by distinct mechanisms. J Biol Chem. 1991 Jun 15;266(17):11025–11029. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth D. D., McCarrey J. R. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Appl. 1992 May;1(4):279–282. doi: 10.1101/gr.1.4.279. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Tobler P. H., Kaufmann M., Born W., Henke H., Cooper P. E., Sagar S. M., Martin J. B. Calcitonin: regional distribution of the hormone and its binding sites in the human brain and pituitary. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7801–7805. doi: 10.1073/pnas.78.12.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchereau-Peron M., Moukhtar M. S., Benson A. A., Milhaud G. Characterization of specific receptors for calcitonin in porcine lung. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3973–3975. doi: 10.1073/pnas.78.6.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Raisz L. G. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science. 1965 Dec 10;150(3702):1465–1467. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Petrison K. K., Bhan A. K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987 Feb;79(2):483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S. R., Schiller A. L., Mankin H. J., Dayer J. M., Krane S. M. Characterization of cells from human giant cell tumors of bone. Clin Orthop Relat Res. 1986 Mar;(204):59–75. [PubMed] [Google Scholar]
- Goltzman D., Mitchell J. Interaction of calcitonin and calcitonin gene-related peptide at receptor sites in target tissues. Science. 1985 Mar 15;227(4692):1343–1345. doi: 10.1126/science.2983422. [DOI] [PubMed] [Google Scholar]
- Gorn A. H., Lin H. Y., Yamin M., Auron P. E., Flannery M. R., Tapp D. R., Manning C. A., Lodish H. F., Krane S. M., Goldring S. R. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest. 1992 Nov;90(5):1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano M., Colucci S., De Bellis M., Zigrino P., Argentino L., Zambonin G., Serra M., Scotlandi K., Teti A., Zambonin Zallone A. New model for bone resorption study in vitro: human osteoclast-like cells from giant cell tumors of bone. J Bone Miner Res. 1994 Jul;9(7):1013–1020. doi: 10.1002/jbmr.5650090708. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Human fetal osteoclasts fail to express macrophage antigens. Br J Exp Pathol. 1985 Feb;66(1):103–108. [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jelinek L. J., Lok S., Rosenberg G. B., Smith R. A., Grant F. J., Biggs S., Bensch P. A., Kuijper J. L., Sheppard P. O., Sprecher C. A. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993 Mar 12;259(5101):1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- Joyner C. J., Quinn J. M., Triffitt J. T., Owen M. E., Athanasou N. A. Phenotypic characterisation of mononuclear and multinucleated cells of giant cell tumour of bone. Bone Miner. 1992 Jan;16(1):37–48. doi: 10.1016/0169-6009(92)90820-4. [DOI] [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991 Nov 15;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., O'Brien J. S. Direct sequencing from low-melt agarose with Sequenase. Nucleic Acids Res. 1989 Jul 25;17(14):5864–5864. doi: 10.1093/nar/17.14.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., Arendt A., McDowell J. H., Kahlert M., Hargrave P. A., Hofmann K. P. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Lin S. C., Chang C. P., Rosenfeld M. G. Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature. 1992 Dec 24;360(6406):765–768. doi: 10.1038/360765a0. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Luttrell L. M., Ostrowski J., Cotecchia S., Kendall H., Lefkowitz R. J. Antagonism of catecholamine receptor signaling by expression of cytoplasmic domains of the receptors. Science. 1993 Mar 5;259(5100):1453–1457. doi: 10.1126/science.8383880. [DOI] [PubMed] [Google Scholar]
- Mayo K. E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992 Oct;6(10):1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- Moro O., Lameh J., Högger P., Sadée W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993 Oct 25;268(30):22273–22276. [PubMed] [Google Scholar]
- Morton C. C., Kirsch I. R., Taub R., Orkin S. H., Brown J. A. Localization of the beta-globin gene by chromosomal in situ hybridization. Am J Hum Genet. 1984 May;36(3):576–585. [PMC free article] [PubMed] [Google Scholar]
- Münch G., Dees C., Hekman M., Palm D. Multisite contacts involved in coupling of the beta-adrenergic receptor with the stimulatory guanine-nucleotide-binding regulatory protein. Structural and functional studies by beta-receptor-site-specific synthetic peptides. Eur J Biochem. 1991 Jun 1;198(2):357–364. doi: 10.1111/j.1432-1033.1991.tb16023.x. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., D'Santos C. S., Evans T., Moseley J. M., Kemp B. E., Michelangeli V. P., Martin T. J. Human placental calcitonin receptors. Biochem J. 1988 Mar 15;250(3):877–882. doi: 10.1042/bj2500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A., Shigemoto R., Nakamura Y., Okamoto N., Mizuno N., Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994 May 6;77(3):361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Regan J. W., Leader W. M., Caron M. G., Lefkowitz R. J. Site-directed mutagenesis of the cytoplasmic domains of the human beta 2-adrenergic receptor. Localization of regions involved in G protein-receptor coupling. J Biol Chem. 1988 Nov 5;263(31):15985–15992. [PubMed] [Google Scholar]
- Okamoto N., Hori S., Akazawa C., Hayashi Y., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994 Jan 14;269(2):1231–1236. [PubMed] [Google Scholar]
- Okamoto T., Murayama Y., Hayashi Y., Inagaki M., Ogata E., Nishimoto I. Identification of a Gs activator region of the beta 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell. 1991 Nov 15;67(4):723–730. doi: 10.1016/0092-8674(91)90067-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and alpha 2-adrenergic receptors based upon characteristics in primary structure. J Biol Chem. 1992 Apr 25;267(12):8342–8346. [PubMed] [Google Scholar]
- Oursler M. J., Collin-Osdoby P., Anderson F., Li L., Webber D., Osdoby P. Isolation of avian osteoclasts: improved techniques to preferentially purify viable cells. J Bone Miner Res. 1991 Apr;6(4):375–385. doi: 10.1002/jbmr.5650060409. [DOI] [PubMed] [Google Scholar]
- Reagan J. D. Expression cloning of an insect diuretic hormone receptor. A member of the calcitonin/secretin receptor family. J Biol Chem. 1994 Jan 7;269(1):9–12. [PubMed] [Google Scholar]
- Roth M. G., Gundersen D., Patil N., Rodriguez-Boulan E. The large external domain is sufficient for the correct sorting of secreted or chimeric influenza virus hemagglutinins in polarized monkey kidney cells. J Cell Biol. 1987 Mar;104(3):769–782. doi: 10.1083/jcb.104.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton P. M., Houssami S., Hilton J. M., O'Keeffe L. M., Center R. J., Gillespie M. T., Darcy P., Findlay D. M. Identification of brain isoforms of the rat calcitonin receptor. Mol Endocrinol. 1993 Jun;7(6):815–821. doi: 10.1210/mend.7.6.8395656. [DOI] [PubMed] [Google Scholar]
- Silvestroni L., Menditto A., Frajese G., Gnessi L. Identification of calcitonin receptors in human spermatozoa. J Clin Endocrinol Metab. 1987 Oct;65(4):742–746. doi: 10.1210/jcem-65-4-742. [DOI] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin T. B., Mezey E., Button D. C., Brownstein M. J., Bonner T. I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993 Dec;133(6):2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- Voss T., Wallner E., Czernilofsky A. P., Freissmuth M. Amphipathic alpha-helical structure does not predict the ability of receptor-derived synthetic peptides to interact with guanine nucleotide-binding regulatory proteins. J Biol Chem. 1993 Mar 5;268(7):4637–4642. [PubMed] [Google Scholar]
- Warshawsky H., Goltzman D., Rouleau M. F., Bergeron J. J. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980 Jun;85(3):682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. R., Kelleher D. J., Johnson G. L. Mapping sites of interaction between rhodopsin and transducin using rhodopsin antipeptide antibodies. J Biol Chem. 1988 May 5;263(13):6150–6154. [PubMed] [Google Scholar]
- Yamin M., Gorn A. H., Flannery M. R., Jenkins N. A., Gilbert D. J., Copeland N. G., Tapp D. R., Krane S. M., Goldring S. R. Cloning and characterization of a mouse brain calcitonin receptor complementary deoxyribonucleic acid and mapping of the calcitonin receptor gene. Endocrinology. 1994 Dec;135(6):2635–2643. doi: 10.1210/endo.135.6.7988453. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S., Cron P., Solinas-Toldo S., Fries R., Lin H. Y., Hemmings B. A. Isolation, characterization, and chromosomal localization of the porcine calcitonin receptor gene. Identification of two variants of the receptor generated by alternative splicing. J Biol Chem. 1994 Jul 29;269(30):19530–19538. [PubMed] [Google Scholar]