Abstract
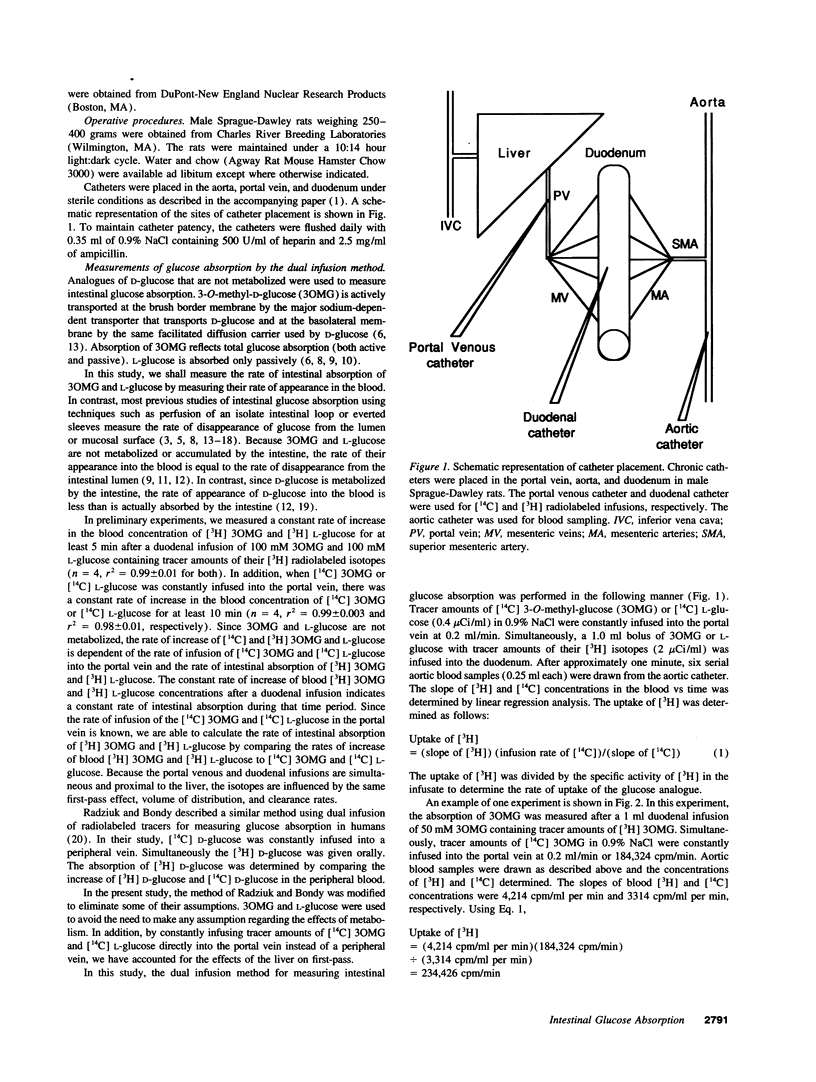
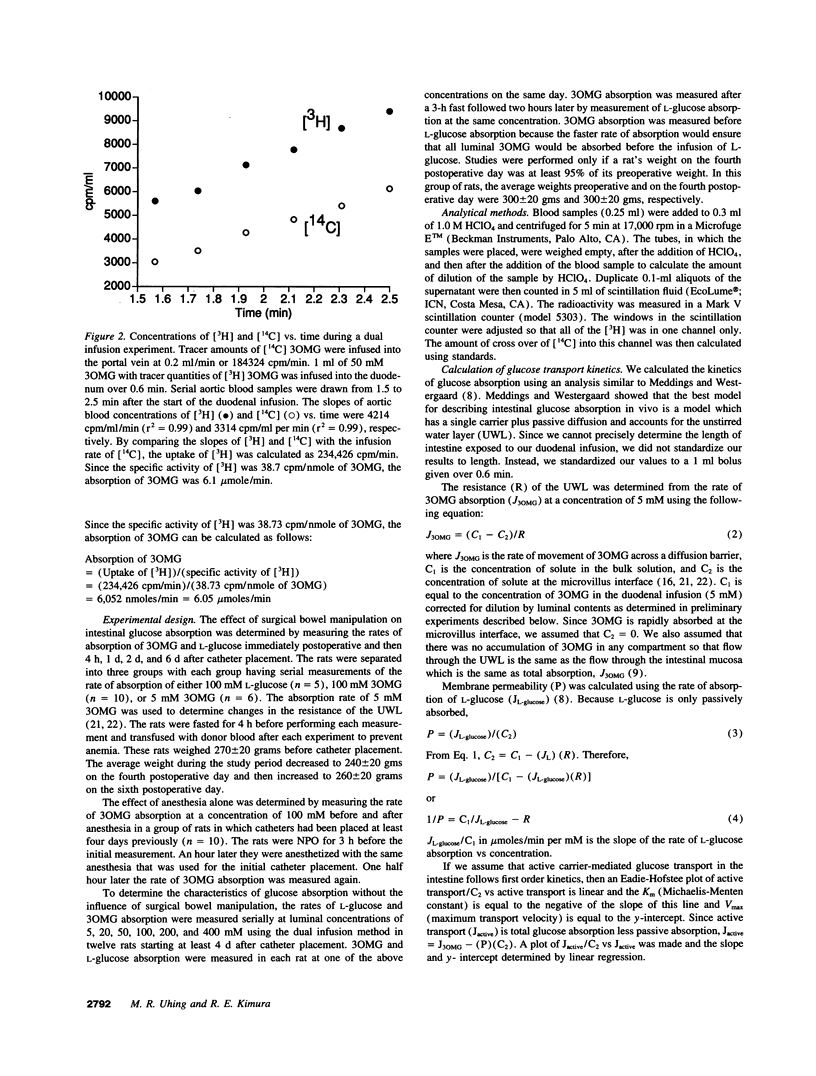
The effects of surgical bowel manipulation and anesthesia on intestinal glucose absorption were determined in chronically catheterized rats. Total and passive rates of glucose absorption were measured using 3-O-methyl-glucose (3OMG) and L-glucose, metabolically inert analogues of D-glucose. The rates of 3OMG absorption immediately postoperative and 4 h later were 86 and 62% less than the absorption rate 6 d postoperative. The absorption rates of 3OMG 1 and 2 d postoperative were not different from 6 d postoperative. Absorption of L-glucose was not altered by bowel manipulation and anesthesia. Even after correction for the increased resistance of the unstirred water layer (UWL) after bowel manipulation, the rates of total and active intestinal glucose absorption immediately postoperative were only 11 and 15% of predicted rates of absorption. In chronically catheterized rats, > 75% of luminal 3OMG at a concentration of 400 mM was absorbed by active transport. The Km and Vmax of 3OMG active transport corrected for the resistance of the UWL were 11.3 mM and 15.6 mumoles/min, respectively. We conclude that measurements of intestinal glucose absorption performed within 24 h of surgical bowel manipulation greatly underestimate active absorption even if corrections are made to account for the increased resistance of the UWL.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. W., Levine A. S., Levitt D. G., Kneip J. M., Levitt M. D. Physiological measurement of luminal stirring in perfused rat jejunum. Am J Physiol. 1988 Jun;254(6 Pt 1):G843–G848. doi: 10.1152/ajpgi.1988.254.6.G843. [DOI] [PubMed] [Google Scholar]
- Atisook K., Carlson S., Madara J. L. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol. 1990 Jan;258(1 Pt 1):C77–C85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- Biolo G., Tessari P., Inchiostro S., Bruttomesso D., Fongher C., Sabadin L., Fratton M. G., Valerio A., Tiengo A. Leucine and phenylalanine kinetics during mixed meal ingestion: a multiple tracer approach. Am J Physiol. 1992 Apr;262(4 Pt 1):E455–E463. doi: 10.1152/ajpendo.1992.262.4.E455. [DOI] [PubMed] [Google Scholar]
- Boyd C. A., Parsons D. S. Effects of vascular perfusion on the accumulation, distribution and transfer of 3-O-methyl-D-glucose within and across the small intestine. J Physiol. 1978 Jan;274:17–36. doi: 10.1113/jphysiol.1978.sp012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddington R. K., Diamond J. Ontogenetic development of nutrient transporters in cat intestine. Am J Physiol. 1992 Nov;263(5 Pt 1):G605–G616. doi: 10.1152/ajpgi.1992.263.5.G605. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., THALE M. Effect of ionic environment on intestinal sugar transport. J Physiol. 1960 Apr;151:59–65. [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I., Harley B. Adaptation of glucose transport across rat enterocyte basolateral membrane in response to altered dietary carbohydrate intake. J Physiol. 1991 Jun;437:563–575. doi: 10.1113/jphysiol.1991.sp018611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Karasov W. H., Thompson C. S. Nutrient uptake by rat enterocytes during diabetes mellitus; evidence for an increased sodium electrochemical gradient. J Physiol. 1988 Mar;397:503–512. doi: 10.1113/jphysiol.1988.sp017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975 Mar;246(1):181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak R. N., Chang E. B., Madara J. L., Field M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J Clin Invest. 1987 Jun;79(6):1571–1578. doi: 10.1172/JCI112991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak R. N., Thomson A. B., Porter V. M. Adaptation of intestinal glucose transport in rats with diabetes mellitus occurs independent of hyperphagia. Can J Physiol Pharmacol. 1991 Aug;69(8):1143–1148. doi: 10.1139/y91-167. [DOI] [PubMed] [Google Scholar]
- Fine K. D., Santa Ana C. A., Porter J. L., Fordtran J. S. Effect of D-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology. 1993 Oct;105(4):1117–1125. doi: 10.1016/0016-5085(93)90957-e. [DOI] [PubMed] [Google Scholar]
- Gumbleton M., Nicholls P. J., Taylor G. Differential influence of laboratory anaesthetic regimens upon renal and hepatosplanchnic haemodynamics in the rat. J Pharm Pharmacol. 1990 Oct;42(10):693–697. doi: 10.1111/j.2042-7158.1990.tb06561.x. [DOI] [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Harris M. S., Kennedy J. G. Relationship between distention and absorption in rat intestine. II. Effects of volume and flow rate on transport. Gastroenterology. 1988 May;94(5 Pt 1):1172–1179. doi: 10.1016/0016-5085(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Harris M. S., Ramaswamy K., Kennedy J. G. Induction of neurally mediated NaHCO3 secretion by luminal distension in rat ileum. Am J Physiol. 1989 Aug;257(2 Pt 1):G191–G197. doi: 10.1152/ajpgi.1989.257.2.G191. [DOI] [PubMed] [Google Scholar]
- Hollander D., Koyama S., Dadufalza V., Tran D. Q., Krugliak P., Ma T., Ling K. Y. Polyethylene glycol 900 permeability of rat intestinal and colonic segments in vivo and brush border membrane vesicles in vitro. J Lab Clin Med. 1989 Apr;113(4):505–515. [PubMed] [Google Scholar]
- Karasov W. H., Debnam E. S. Rapid adaptation of intestinal glucose transport: a brush-border or basolateral phenomenon? Am J Physiol. 1987 Jul;253(1 Pt 1):G54–G61. doi: 10.1152/ajpgi.1987.253.1.G54. [DOI] [PubMed] [Google Scholar]
- Karbach U., Wanitschke R. Influence of serosal hydrostatic pressure on net water and electrolyte transport across the isolated rat colonic mucosa exposed to different secretagogues. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):336–341. doi: 10.1007/BF00506246. [DOI] [PubMed] [Google Scholar]
- Kimura R. E., LaPine T. R., Gooch W. M., 3rd Portal venous and aortic glucose and lactate changes in a chronically catheterized rat. Pediatr Res. 1988 Feb;23(2):235–240. doi: 10.1203/00006450-198802000-00021. [DOI] [PubMed] [Google Scholar]
- Kimura R. E., Thulin G., Warshaw J. B. The effect of ketone bodies and fatty acid on intestinal glucose metabolism during development. Pediatr Res. 1984 Jul;18(7):575–578. doi: 10.1203/00006450-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Furne J. K., Levitt D. G. Shaking of the intact rat and intestinal angulation diminish the jejunal unstirred layer. Gastroenterology. 1992 Nov;103(5):1460–1466. doi: 10.1016/0016-5085(92)91165-z. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Furne J. K., Strocchi A., Anderson B. W., Levitt D. G. Physiological measurements of luminal stirring in the dog and human small bowel. J Clin Invest. 1990 Nov;86(5):1540–1547. doi: 10.1172/JCI114873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Pappenheimer J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Malo C., Berteloot A. Analysis of kinetic data in transport studies: new insights from kinetic studies of Na(+)-D-glucose cotransport in human intestinal brush-border membrane vesicles using a fast sampling, rapid filtration apparatus. J Membr Biol. 1991 Jun;122(2):127–141. doi: 10.1007/BF01872636. [DOI] [PubMed] [Google Scholar]
- Meddings J. B., DeSouza D., Goel M., Thiesen S. Glucose transport and microvillus membrane physical properties along the crypt-villus axis of the rabbit. J Clin Invest. 1990 Apr;85(4):1099–1107. doi: 10.1172/JCI114541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddings J. B., Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin Sci (Lond) 1989 Apr;76(4):403–413. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- Moe A. J., Jackson M. J. Isolation and characterization of brush border membrane vesicles from pig small intestine. Comp Biochem Physiol A Comp Physiol. 1987;88(3):511–517. doi: 10.1016/0300-9629(87)90073-9. [DOI] [PubMed] [Google Scholar]
- Murakami E., Saito M., Suda M. Contribution of diffusive pathway in intestinal absorption of glucose in rat under normal feeding condition. Experientia. 1977 Nov 15;33(11):1469–1470. doi: 10.1007/BF01918813. [DOI] [PubMed] [Google Scholar]
- Nicholls T. J., Leese H. J., Bronk J. R. Transport and metabolism of glucose by rat small intestine. Biochem J. 1983 Apr 15;212(1):183–187. doi: 10.1042/bj2120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer J. R., Reiss K. Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Bondy D. C. Abnormal oral glucose tolerance and glucose malabsorption after vagotomy and pyloroplasty. A tracer method for measuring glucose absorption rates. Gastroenterology. 1982 Nov;83(5):1017–1025. [PubMed] [Google Scholar]
- Rich-Denson C., Kimura R. E. Evidence in vivo that most of the intraluminally absorbed glucose is absorbed intact into the portal vein and not metabolized to lactate. Biochem J. 1988 Sep 15;254(3):931–934. doi: 10.1042/bj2540931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Chaudry K. I., Chudler L. C., O'Neill P. J., Chaudry I. H. Measurement of D-xylose gut absorptive capacity in conscious rats. Am J Physiol. 1991 Nov;261(5 Pt 2):R1313–R1320. doi: 10.1152/ajpregu.1991.261.5.R1313. [DOI] [PubMed] [Google Scholar]
- Sparsø B. H., Luke M., Wium E. Electrogenic transport of glucose in the normal upper duodenum. II. Unstirred water layer and estimation of real transport constants. Scand J Gastroenterol. 1984 Jun;19(4):568–574. [PubMed] [Google Scholar]
- Thomson A. B., Hotke C. A., Weinstein W. M. Comparison of kinetic constants of hexose uptake in four animal species and man. Comp Biochem Physiol A Comp Physiol. 1982;72(1):225–236. doi: 10.1016/0300-9629(82)90037-8. [DOI] [PubMed] [Google Scholar]
- Uhing M. R., Kimura R. E. Active transport of 3-O-methyl-glucose by the small intestine in chronically catheterized rats. J Clin Invest. 1995 Jun;95(6):2799–2805. doi: 10.1172/JCI117984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., LANDAU B. R. Specificity of sugar transport by the intestine of the hamster. Am J Physiol. 1960 Jan;198:99–102. doi: 10.1152/ajplegacy.1960.198.1.99. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Holtermüller K. H., Dietschy J. M. Measurement of resistance of barriers to solute transport in vivo in rat jejunum. Am J Physiol. 1986 Jun;250(6 Pt 1):G727–G735. doi: 10.1152/ajpgi.1986.250.6.G727. [DOI] [PubMed] [Google Scholar]
- Westergaard H. Insulin modulates rat intestinal glucose transport: effect of hypoinsulinemia and hyperinsulinemia. Am J Physiol. 1989 May;256(5 Pt 1):G911–G918. doi: 10.1152/ajpgi.1989.256.5.G911. [DOI] [PubMed] [Google Scholar]


