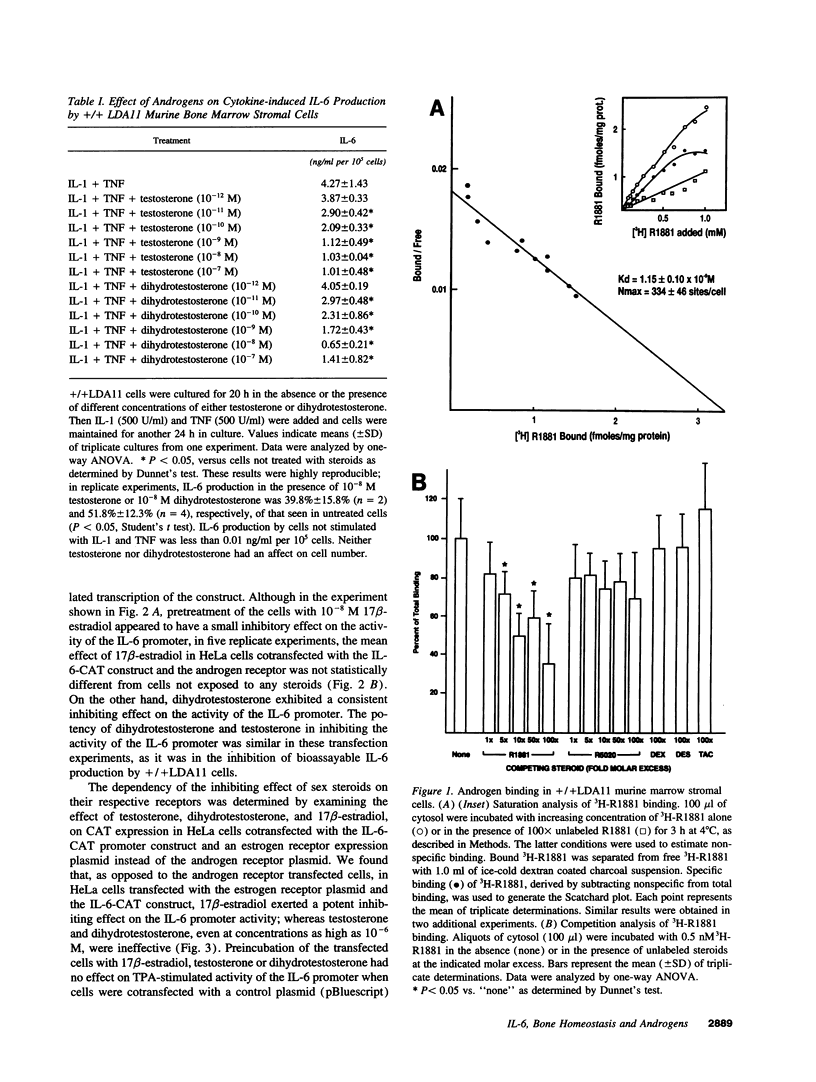
Abstract
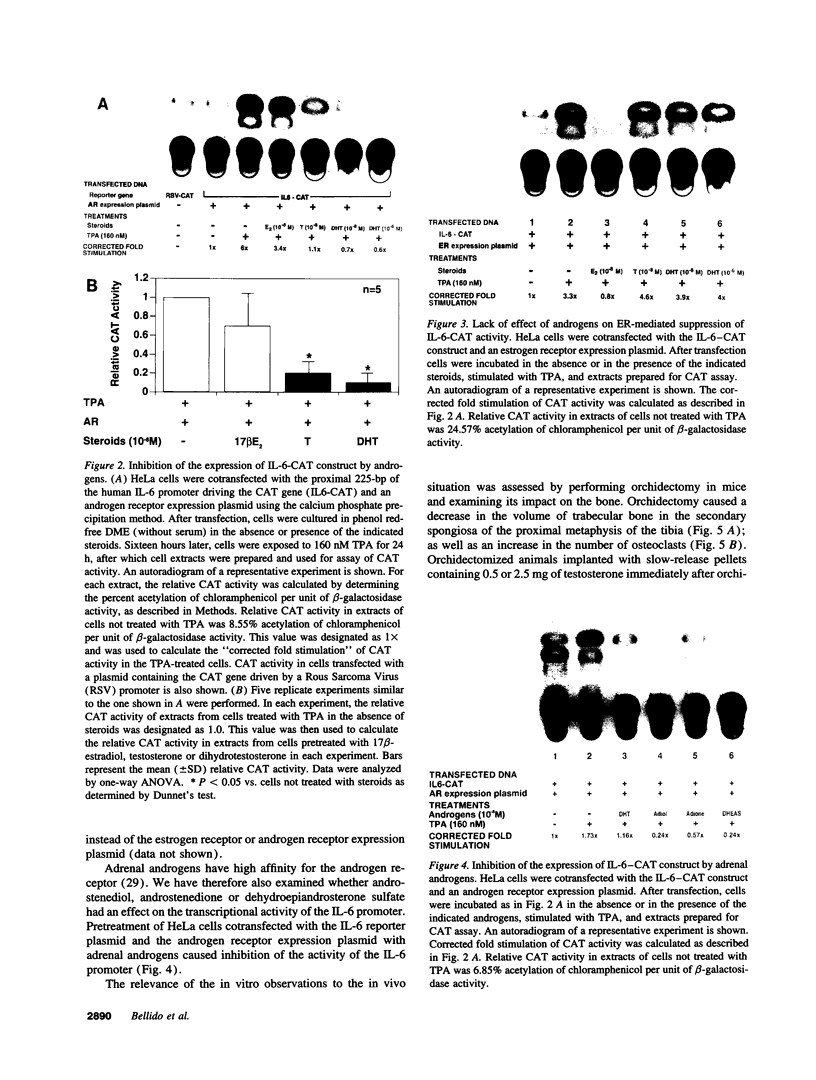
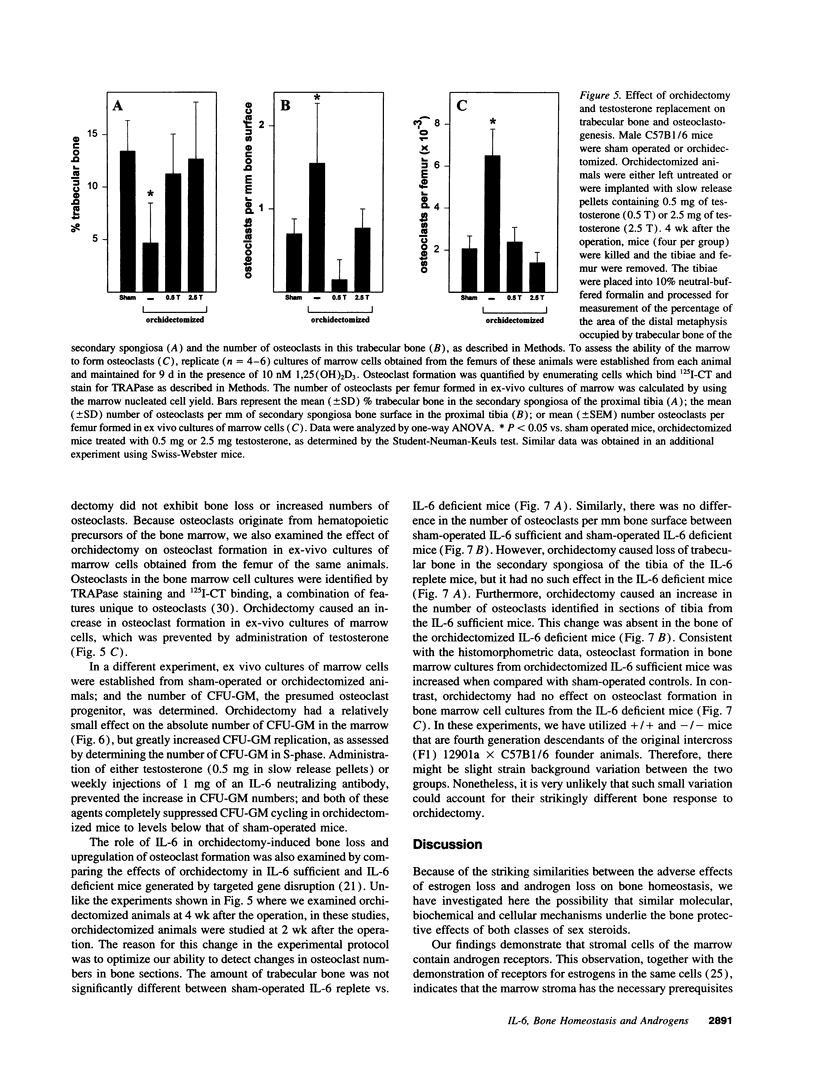
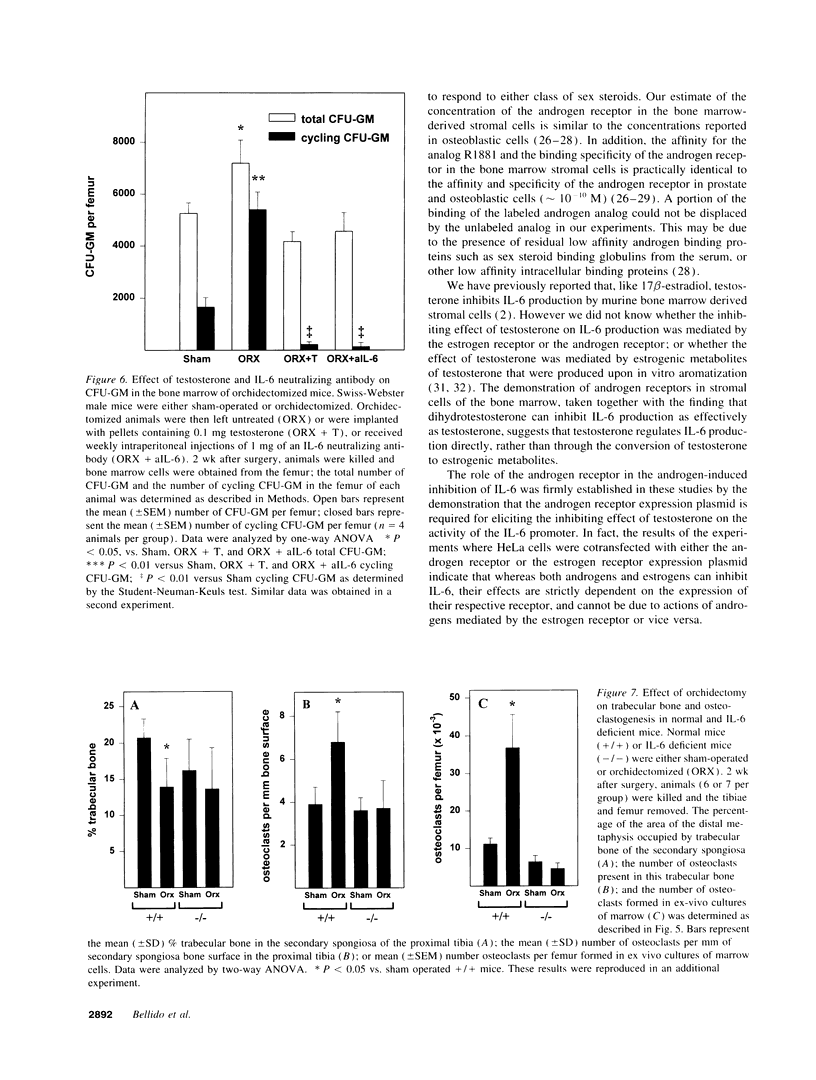
Interleukin-6 is an essential mediator of the bone loss caused by loss of estrogens. Because loss of androgens also causes bone loss, we have examined whether the IL-6 gene is regulated by androgens, and whether IL-6 plays a role in the bone loss caused by androgen deficiency. Both testosterone and dihydrotestosterone inhibited IL-6 production by murine bone marrow-derived stromal cells. In addition, testosterone, dihydrotestosterone, and adrenal androgens inhibited the expression of a chloramphenicol acetyl transferase reporter plasmid driven by the human IL-6 promoter in HeLa cells cotransfected with an androgen receptor expression plasmid; however, these steroids were ineffective when the cells were cotransfected with an estrogen receptor expression plasmid. In accordance with the in vitro findings, orchidectomy in mice caused an increase in the replication of osteoclast progenitors in the bone marrow which could be prevented by androgen replacement or administration of an IL-6 neutralizing antibody. Moreover, bone histomorphometric analysis of trabecular bone revealed that, in contrast to IL-6 sufficient mice which exhibited increased osteoclast numbers and bone loss following orchidectomy, IL-6 deficient mice (generated by targeted gene disruption) did not. This evidence demonstrates that male sex steroids, acting through the androgen-specific receptor, inhibit the expression of the IL-6 gene; and that IL-6 mediates the upregulation of osteoclastogenesis and therefore the bone loss caused by androgen deficiency, as it does in estrogen deficiency.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellido T., Girasole G., Passeri G., Yu X. P., Mocharla H., Jilka R. L., Notides A., Manolagas S. C. Demonstration of estrogen and vitamin D receptors in bone marrow-derived stromal cells: up-regulation of the estrogen receptor by 1,25-dihydroxyvitamin-D3. Endocrinology. 1993 Aug;133(2):553–562. doi: 10.1210/endo.133.2.8393768. [DOI] [PubMed] [Google Scholar]
- Benz D. J., Haussler M. R., Thomas M. A., Speelman B., Komm B. S. High-affinity androgen binding and androgenic regulation of alpha 1(I)-procollagen and transforming growth factor-beta steady state messenger ribonucleic acid levels in human osteoblast-like osteosarcoma cells. Endocrinology. 1991 Jun;128(6):2723–2730. doi: 10.1210/endo-128-6-2723. [DOI] [PubMed] [Google Scholar]
- Black K., Garrett I. R., Mundy G. R. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene cause hypercalcemia as well as cachexia, leukocytosis and thrombocytosis in tumor-bearing nude mice. Endocrinology. 1991 May;128(5):2657–2659. doi: 10.1210/endo-128-5-2657. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Hangoc G., Cooper S., Gentile P., Shen R. N., Ralph P., Gillis S., Bicknell D. C. The opposing actions in vivo on murine myelopoiesis of purified preparations of lactoferrin and the colony stimulating factors. Blood Cells. 1987;13(1-2):31–48. [PubMed] [Google Scholar]
- Claessens F., Celis L., Peeters B., Heyns W., Verhoeven G., Rombauts W. Functional characterization of an androgen response element in the first intron of the C3(1) gene of prostatic binding protein. Biochem Biophys Res Commun. 1989 Oct 31;164(2):833–840. doi: 10.1016/0006-291x(89)91534-9. [DOI] [PubMed] [Google Scholar]
- Colvard D. S., Eriksen E. F., Keeting P. E., Wilson E. M., Lubahn D. B., French F. S., Riggs B. L., Spelsberg T. C. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):854–857. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb D. W., Minth C. D., Dixon J. E. Assaying the reporter gene chloramphenicol acetyltransferase. Methods Enzymol. 1989;168:690–701. doi: 10.1016/0076-6879(89)68050-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Finkelstein J. S., Klibanski A., Neer R. M., Doppelt S. H., Rosenthal D. I., Segre G. V., Crowley W. F., Jr Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1989 Oct;69(4):776–783. doi: 10.1210/jcem-69-4-776. [DOI] [PubMed] [Google Scholar]
- Flynn A. Expression of Ia and the production of interleukin 1 by peritoneal exudate macrophages activated in vivo by steroids. Life Sci. 1986 Jun 30;38(26):2455–2460. doi: 10.1016/0024-3205(86)90616-8. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girasole G., Passeri G., Jilka R. L., Manolagas S. C. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest. 1994 Apr;93(4):1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldray D., Weisman Y., Jaccard N., Merdler C., Chen J., Matzkin H. Decreased bone density in elderly men treated with the gonadotropin-releasing hormone agonist decapeptyl (D-Trp6-GnRH). J Clin Endocrinol Metab. 1993 Feb;76(2):288–290. doi: 10.1210/jcem.76.2.7679397. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Greenspan S. L., Neer R. M., Ridgway E. C., Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1986 Jun;104(6):777–782. doi: 10.7326/0003-4819-104-6-777. [DOI] [PubMed] [Google Scholar]
- Grino P. B., Griffin J. E., Wilson J. D. Transformation of the androgen receptor to the deoxyribonucleic acid-binding state: studies in homogenates and intact cells. Endocrinology. 1987 May;120(5):1914–1920. doi: 10.1210/endo-120-5-1914. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Hu S. K., Mitcho Y. L., Rath N. C. Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol. 1988;10(3):247–252. doi: 10.1016/0192-0561(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Hustmyer F. G., Walker E., Yu X. P., Girasole G., Sakagami Y., Peacock M., Manolagas S. C. Cytokine production and surface antigen expression by peripheral blood mononuclear cells in postmenopausal osteoporosis. J Bone Miner Res. 1993 Jan;8(1):51–59. doi: 10.1002/jbmr.5650080108. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Miyaura C., Jin C. H., Akatsu T., Abe E., Nakamura Y., Yamaguchi A., Yoshiki S., Matsuda T., Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990 Nov 15;145(10):3297–3303. [PubMed] [Google Scholar]
- Jackson J. A., Riggs M. W., Spiekerman A. M. Testosterone deficiency as a risk factor for hip fractures in men: a case-control study. Am J Med Sci. 1992 Jul;304(1):4–8. doi: 10.1097/00000441-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kalu D. N., Salerno E., Liu C. C., Ferarro F., Arjmandi B. N., Salih M. A. Ovariectomy-induced bone loss and the hematopoietic system. Bone Miner. 1993 Nov;23(2):145–161. doi: 10.1016/s0169-6009(08)80050-5. [DOI] [PubMed] [Google Scholar]
- Kimble R. B., Vannice J. L., Bloedow D. C., Thompson R. C., Hopfer W., Kung V. T., Brownfield C., Pacifici R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994 May;93(5):1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa R., Kimble R. B., Vannice J. L., Kung V. T., Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994 Dec;94(6):2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty K. W., Christensen R. D. In vivo effect of interleukin-6 on cycling status of hematopoietic progenitors from adults and neonates. Pediatr Res. 1990 Oct;28(4):323–326. doi: 10.1203/00006450-199010000-00004. [DOI] [PubMed] [Google Scholar]
- Löwik C. W., van der Pluijm G., Bloys H., Hoekman K., Bijvoet O. L., Aarden L. A., Papapoulos S. E. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogenesis. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- Manolagas S. C., Jilka R. L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995 Feb 2;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Maze R., Sherry B., Kwon B. S., Cerami A., Broxmeyer H. E. Myelosuppressive effects in vivo of purified recombinant murine macrophage inflammatory protein-1 alpha. J Immunol. 1992 Aug 1;149(3):1004–1009. [PubMed] [Google Scholar]
- Murphy S., Khaw K. T., Cassidy A., Compston J. E. Sex hormones and bone mineral density in elderly men. Bone Miner. 1993 Feb;20(2):133–140. doi: 10.1016/s0169-6009(08)80022-0. [DOI] [PubMed] [Google Scholar]
- Nordin B. E., Robertson A., Seamark R. F., Bridges A., Philcox J. C., Need A. G., Horowitz M., Morris H. A., Deam S. The relation between calcium absorption, serum dehydroepiandrosterone, and vertebral mineral density in postmenopausal women. J Clin Endocrinol Metab. 1985 Apr;60(4):651–657. doi: 10.1210/jcem-60-4-651. [DOI] [PubMed] [Google Scholar]
- Orwoll E. S., Stribrska L., Ramsey E. E., Keenan E. J. Androgen receptors in osteoblast-like cell lines. Calcif Tissue Int. 1991 Sep;49(3):183–187. doi: 10.1007/BF02556115. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., McCracken R., Vered I., McMurtry C., Avioli L. V., Peck W. A. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri G., Girasole G., Jilka R. L., Manolagas S. C. Increased interleukin-6 production by murine bone marrow and bone cells after estrogen withdrawal. Endocrinology. 1993 Aug;133(2):822–828. doi: 10.1210/endo.133.2.8393776. [DOI] [PubMed] [Google Scholar]
- Pojda Z., Tsuboi A. In vivo effects of human recombinant interleukin 6 on hemopoietic stem and progenitor cells and circulating blood cells in normal mice. Exp Hematol. 1990 Oct;18(9):1034–1037. [PubMed] [Google Scholar]
- Poli V., Balena R., Fattori E., Markatos A., Yamamoto M., Tanaka H., Ciliberto G., Rodan G. A., Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994 Mar 1;13(5):1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz S. T., Bellido T., Mocharla H., Crabb D., Manolagas S. C. 17 beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994 Mar;93(3):944–950. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A., Flanagan A. M., Reed M. J. Estrogen synthesis by osteoblast cell lines. Endocrinology. 1992 Oct;131(4):2027–2029. doi: 10.1210/endo.131.4.1396346. [DOI] [PubMed] [Google Scholar]
- Ray A., Prefontaine K. E., Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994 Apr 29;269(17):12940–12946. [PubMed] [Google Scholar]
- Roche P. J., Hoare S. A., Parker M. G. A consensus DNA-binding site for the androgen receptor. Mol Endocrinol. 1992 Dec;6(12):2229–2235. doi: 10.1210/mend.6.12.1491700. [DOI] [PubMed] [Google Scholar]
- Sambrook P., Birmingham J., Champion D., Kelly P., Kempler S., Freund J., Eisman J. Postmenopausal bone loss in rheumatoid arthritis: effect of estrogens and androgens. J Rheumatol. 1992 Mar;19(3):357–361. [PubMed] [Google Scholar]
- Seeman E., Melton L. J., 3rd, O'Fallon W. M., Riggs B. L. Risk factors for spinal osteoporosis in men. Am J Med. 1983 Dec;75(6):977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Thompson P. W., Perry L. A., McGarrigle H. H., Edwards A. C. The relationship between sex steroids and bone mineral content in women soon after the menopause. Clin Endocrinol (Oxf) 1991 Jan;34(1):37–41. doi: 10.1111/j.1365-2265.1991.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Stanley H. L., Schmitt B. P., Poses R. M., Deiss W. P. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J Am Geriatr Soc. 1991 Aug;39(8):766–771. doi: 10.1111/j.1532-5415.1991.tb02698.x. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Stepán J. J., Lachman M., Zverina J., Pacovský V., Baylink D. J. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1989 Sep;69(3):523–527. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- Tanabe O., Akira S., Kamiya T., Wong G. G., Hirano T., Kishimoto T. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J Immunol. 1988 Dec 1;141(11):3875–3881. [PubMed] [Google Scholar]
- Tanaka S., Haji M., Nishi Y., Yanase T., Takayanagi R., Nawata H. Aromatase activity in human osteoblast-like osteosarcoma cell. Calcif Tissue Int. 1993 Feb;52(2):107–109. doi: 10.1007/BF00308318. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Lifrak E. T., Beckner M., Wakley G. K., Hannon K. S., Parker L. N. Dehydroepiandrosterone reduces cancellous bone osteopenia in ovariectomized rats. Am J Physiol. 1990 Apr;258(4 Pt 1):E673–E677. doi: 10.1152/ajpendo.1990.258.4.E673. [DOI] [PubMed] [Google Scholar]
- Wakley G. K., Schutte H. D., Jr, Hannon K. S., Turner R. T. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. J Bone Miner Res. 1991 Apr;6(4):325–330. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- Wilson E. M., French F. S. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976 Sep 25;251(18):5620–5629. [PubMed] [Google Scholar]
- Zarrabeitia M. T., Riancho J. A., Amado J. A., Napal J., Gonzalez-Macias J. Cytokine production by peripheral blood cells in postmenopausal osteoporosis. Bone Miner. 1991 Aug;14(2):161–167. doi: 10.1016/0169-6009(91)90093-f. [DOI] [PubMed] [Google Scholar]