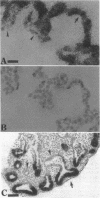
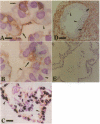
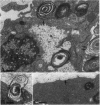
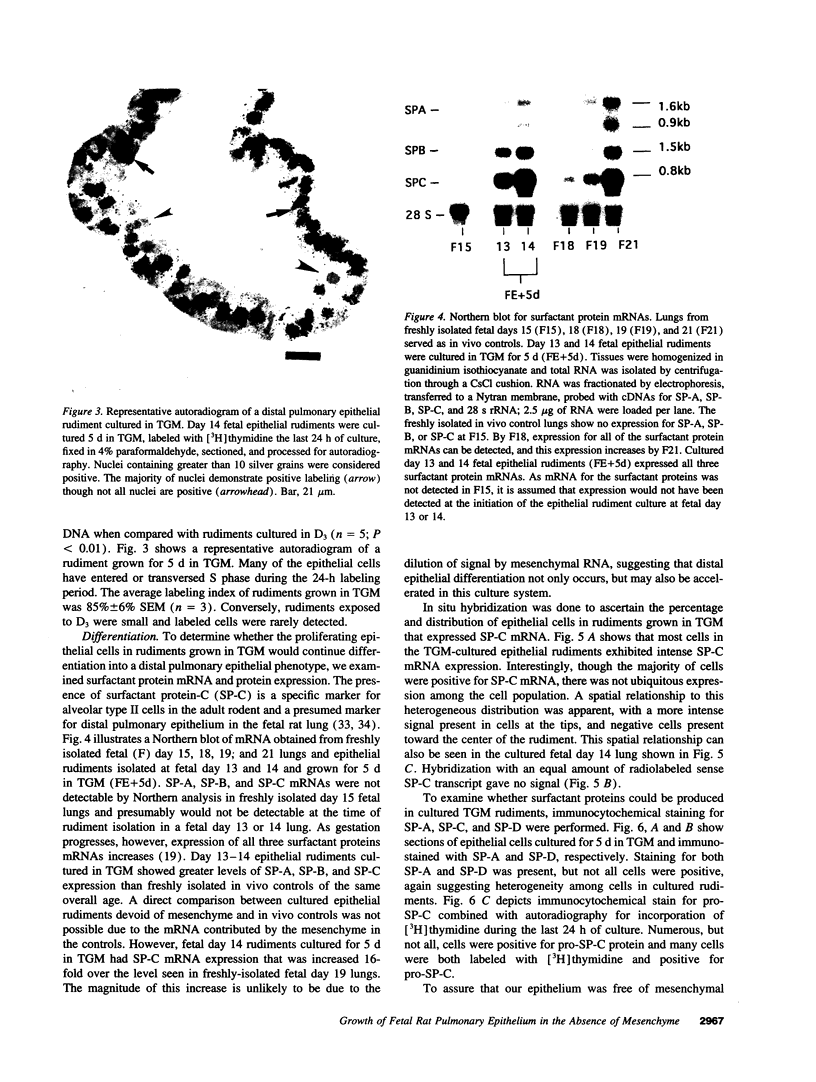
Abstract
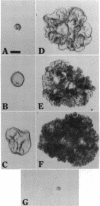
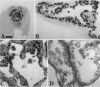
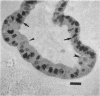
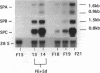
Previous studies have shown that pulmonary mesenchyme is required to maintain epithelial viability and to support branching morphogenesis and cytodifferentiation. We have examined whether pulmonary mesenchyme can be replaced by a medium containing a combination of soluble factors. Day 13-14 fetal rat distal lung epithelium was enzymatically separated from its mesenchyme, enrobed in EHS tumor matrix, and cultured for 5 d in medium containing concentrated bronchoalveolar lavage, EGF, acidic fibroblast growth factor, cholera toxin, insulin, and FBS (TGM), or in control medium containing only FBS. After 5 d in culture, marked growth and morphological changes occurred in epithelial rudiments cultured in TGM, whereas no changes were seen in controls. [3H]Thymidine incorporation and nuclear labeling indices during the last 24 h of culture confirmed that epithelial rudiments cultured in TGM had significant proliferative capacities. Evaluation of surfactant protein gene expression by Northern analysis, in situ hybridization, and immunocytochemistry demonstrated that distal lung epithelial differentiation progressed in TGM. Ultrastructural analysis demonstrated that fetal distal lung epithelium cultured in TGM contained lamellar bodies and deposited a basal lamina. These results are the first demonstration that sustained proliferation and differentiation of glandular stage distal pulmonary epithelium can proceed in the absence of mesenchyme.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg J. J., Otto-Verberne C. J., Ten Have-Opbroek A. A., Klazinga W. Isolation of alveolar type II cells from fetal rat lung by differential adherence in monolayer culture. Biochim Biophys Acta. 1988 Jun 15;960(3):441–453. doi: 10.1016/0005-2760(88)90053-7. [DOI] [PubMed] [Google Scholar]
- Couwenhoven R. I., Snead M. L. Early determination and permissive expression of amelogenin transcription during mouse mandibular first molar development. Dev Biol. 1994 Jul;164(1):290–299. doi: 10.1006/dbio.1994.1199. [DOI] [PubMed] [Google Scholar]
- Deterding R. R., Shimizu H., Fisher J. H., Shannon J. M. Regulation of surfactant protein D expression by glucocorticoids in vitro and in vivo. Am J Respir Cell Mol Biol. 1994 Jan;10(1):30–37. doi: 10.1165/ajrcmb.10.1.8292379. [DOI] [PubMed] [Google Scholar]
- Emrie P. A., Shannon J. M., Mason R. J., Fisher J. H. cDNA and deduced amino acid sequence for the rat hydrophobic pulmonary surfactant-associated protein, SP-B. Biochim Biophys Acta. 1989 Feb 23;994(3):215–221. doi: 10.1016/0167-4838(89)90296-3. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Fisher J. H., Shannon J. M., Hofmann T., Mason R. J. Nucleotide and deduced amino acid sequence of the hydrophobic surfactant protein SP-C from rat: expression in alveolar type II cells and homology with SP-C from other species. Biochim Biophys Acta. 1989 May 1;995(3):225–230. doi: 10.1016/0167-4838(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Fraslon C., Lacaze-Masmonteil T., Zupan V., Chailley-Heu B., Bourbon J. R. Fetal rat lung type II cell differentiation in serum-free isolated cell culture: modulation and inhibition. Am J Physiol. 1993 May;264(5 Pt 1):L504–L516. doi: 10.1152/ajplung.1993.264.5.L504. [DOI] [PubMed] [Google Scholar]
- Ganser G. L., Stricklin G. P., Matrisian L. M. EGF and TGF alpha influence in vitro lung development by the induction of matrix-degrading metalloproteinases. Int J Dev Biol. 1991 Dec;35(4):453–461. [PubMed] [Google Scholar]
- Han R. N., Liu J., Tanswell A. K., Post M. Expression of basic fibroblast growth factor and receptor: immunolocalization studies in developing rat fetal lung. Pediatr Res. 1992 May;31(5):435–440. doi: 10.1203/00006450-199205000-00004. [DOI] [PubMed] [Google Scholar]
- Han R. N., Mawdsley C., Souza P., Tanswell A. K., Post M. Platelet-derived growth factors and growth-related genes in rat lung. III. Immunolocalization during fetal development. Pediatr Res. 1992 Apr;31(4 Pt 1):323–329. doi: 10.1203/00006450-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Jassal D., Han R. N., Caniggia I., Post M., Tanswell A. K. Growth of distal fetal rat lung epithelial cells in a defined serum-free medium. In Vitro Cell Dev Biol. 1991 Aug;27A(8):625–632. doi: 10.1007/BF02631105. [DOI] [PubMed] [Google Scholar]
- Kalina M., Mason R. J., Shannon J. M. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992 Jun;6(6):594–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kresch M. J., Dynia D. W., Gross I. Culture of differentiated and undifferentiated type II cells from fetal rat lung. Biochim Biophys Acta. 1987 Aug 19;930(1):19–32. doi: 10.1016/0167-4889(87)90151-0. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Mason R. J. Bronchoalveolar lavage fluid from normal rats stimulates DNA synthesis in rat alveolar type II cells. Am Rev Respir Dis. 1989 Feb;139(2):360–366. doi: 10.1164/ajrccm/139.2.360. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Mason R. J. Heparin-binding growth factors stimulate DNA synthesis in rat alveolar type II cells. Am J Respir Cell Mol Biol. 1990 Jan;2(1):99–106. doi: 10.1165/ajrcmb/2.1.99. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Mason R. J., Shannon J. M. Proliferation of rat alveolar epithelial cells in low density primary culture. Am J Respir Cell Mol Biol. 1993 Jul;9(1):64–72. doi: 10.1165/ajrcmb/9.1.64. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Robinson P. C., Mason R. J. Stimulation of DNA synthesis in cultured rat alveolar type II cells. Exp Lung Res. 1985;8(1):53–66. doi: 10.3109/01902148509069679. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Fuller-Pace F., Smith R., Dickson C. FGF-7 (keratinocyte growth factor) expression during mouse development suggests roles in myogenesis, forebrain regionalisation and epithelial-mesenchymal interactions. Mech Dev. 1994 Jan;45(1):15–30. doi: 10.1016/0925-4773(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980 Jan 18;617(1):36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Widdicombe J. H., Sanders M. J., Misfeldt D. S., Berry L. C., Jr Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters J. R. Epithelial-mesenchymal interaction during lung development: the effect of mesenchymal mass. Dev Biol. 1976 Jul 1;51(1):98–108. doi: 10.1016/0012-1606(76)90125-1. [DOI] [PubMed] [Google Scholar]
- Miki T., Bottaro D. P., Fleming T. P., Smith C. L., Burgess W. H., Chan A. M., Aaronson S. A. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H., Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991 Jul;112(3):855–861. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A., Bedford M. T., Burakova T., Arman E., Zimmer Y., Yayon A., Givol D., Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev Biol. 1993 Aug;158(2):475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Rubin J. S., Csaky K. G., Aaronson S. A., Mason R. J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993 Aug;92(2):969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Werner S., Liao X., Wert S., Whitsett J., Williams L. Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 1994 Jul 15;13(14):3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Fisher J., Mason R. J., Kuroki Y., Schilling J., Benson B., Voelker D. Isolation and sequence of a cDNA clone for the rat pulmonary surfactant-associated protein (PSP-A). Biochem Biophys Res Commun. 1987 Apr 14;144(1):367–374. doi: 10.1016/s0006-291x(87)80519-3. [DOI] [PubMed] [Google Scholar]
- Schellhase D. E., Emrie P. A., Fisher J. H., Shannon J. M. Ontogeny of surfactant apoproteins in the rat. Pediatr Res. 1989 Sep;26(3):167–174. doi: 10.1203/00006450-198909000-00001. [DOI] [PubMed] [Google Scholar]
- Schellhase D. E., Shannon J. M. Effects of maternal dexamethasone on expression of SP-A, SP-B, and SP-C in the fetal rat lung. Am J Respir Cell Mol Biol. 1991 Apr;4(4):304–312. doi: 10.1165/ajrcmb/4.4.304. [DOI] [PubMed] [Google Scholar]
- Seth R., Shum L., Wu F., Wuenschell C., Hall F. L., Slavkin H. C., Warburton D. Role of epidermal growth factor expression in early mouse embryo lung branching morphogenesis in culture: antisense oligodeoxynucleotide inhibitory strategy. Dev Biol. 1993 Aug;158(2):555–559. doi: 10.1006/dbio.1993.1213. [DOI] [PubMed] [Google Scholar]
- Shannon J. M. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994 Dec;166(2):600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Fisher J. H., Papst P., Benson B., Lau K., Mason R. J., Voelker D. R. Primary structure of rat pulmonary surfactant protein D. cDNA and deduced amino acid sequence. J Biol Chem. 1992 Jan 25;267(3):1853–1857. [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970 Dec;175(4):445–454. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- Stiles A. D., Smith B. T., Post M. Reciprocal autocrine and paracrine regulation of growth of mesenchymal and alveolar epithelial cells from fetal lung. Exp Lung Res. 1986;11(3):165–177. doi: 10.3109/01902148609064294. [DOI] [PubMed] [Google Scholar]
- Taderera J. V. Control of lung differentiation in vitro. Dev Biol. 1967 Nov;16(5):489–512. doi: 10.1016/0012-1606(67)90061-9. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H. K., Luyten F. P., Roberts A. B., Roche N. S., Reddi A. H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992 Sep;202(1):1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Wang J., Souza P., Kuliszewski M., Tanswell A. K., Post M. Expression of surfactant proteins in embryonic rat lung. Am J Respir Cell Mol Biol. 1994 Feb;10(2):222–229. doi: 10.1165/ajrcmb.10.2.7509164. [DOI] [PubMed] [Google Scholar]
- Warburton D., Seth R., Shum L., Horcher P. G., Hall F. L., Werb Z., Slavkin H. C. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev Biol. 1992 Jan;149(1):123–133. doi: 10.1016/0012-1606(92)90269-m. [DOI] [PubMed] [Google Scholar]
- Wert S. E., Glasser S. W., Korfhagen T. R., Whitsett J. A. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993 Apr;156(2):426–443. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- Wohlford-Lenane C. L., Durham P. L., Snyder J. M. Localization of surfactant-associated protein C (SP-C) mRNA in fetal rabbit lung tissue by in situ hybridization. Am J Respir Cell Mol Biol. 1992 Feb;6(2):225–234. doi: 10.1165/ajrcmb/6.2.225. [DOI] [PubMed] [Google Scholar]