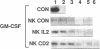Abstract
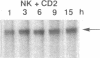
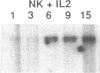
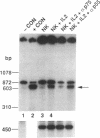
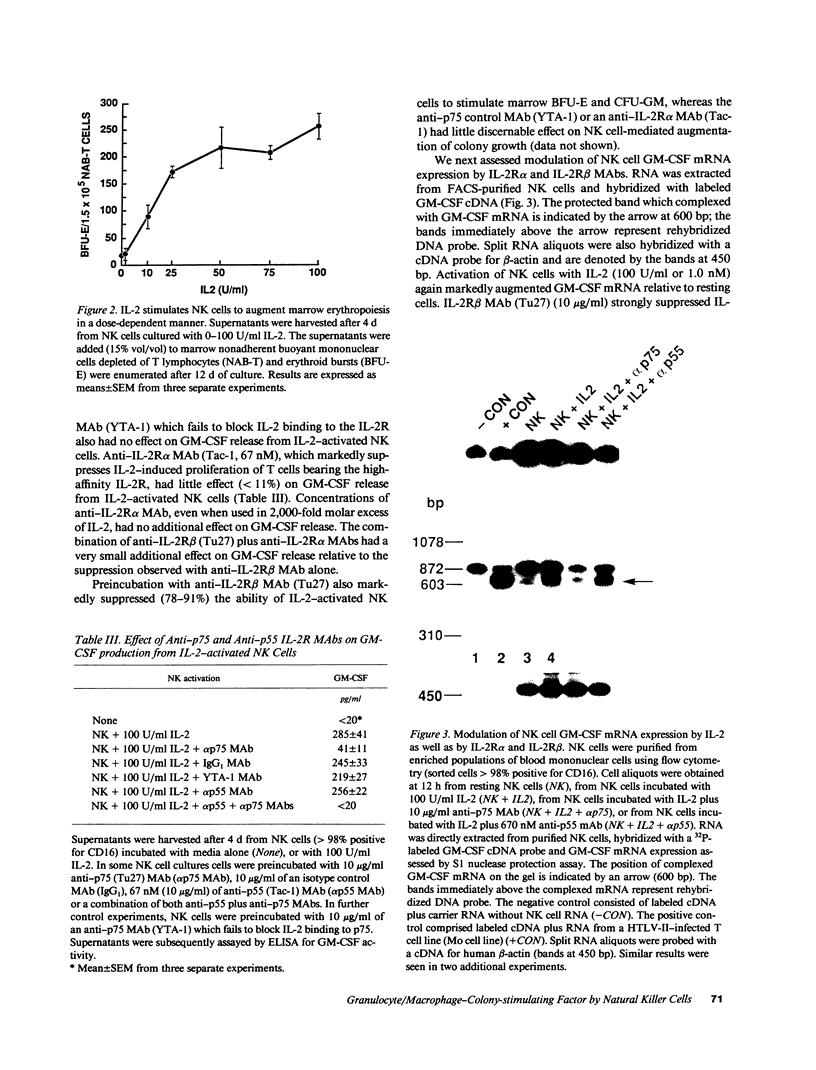
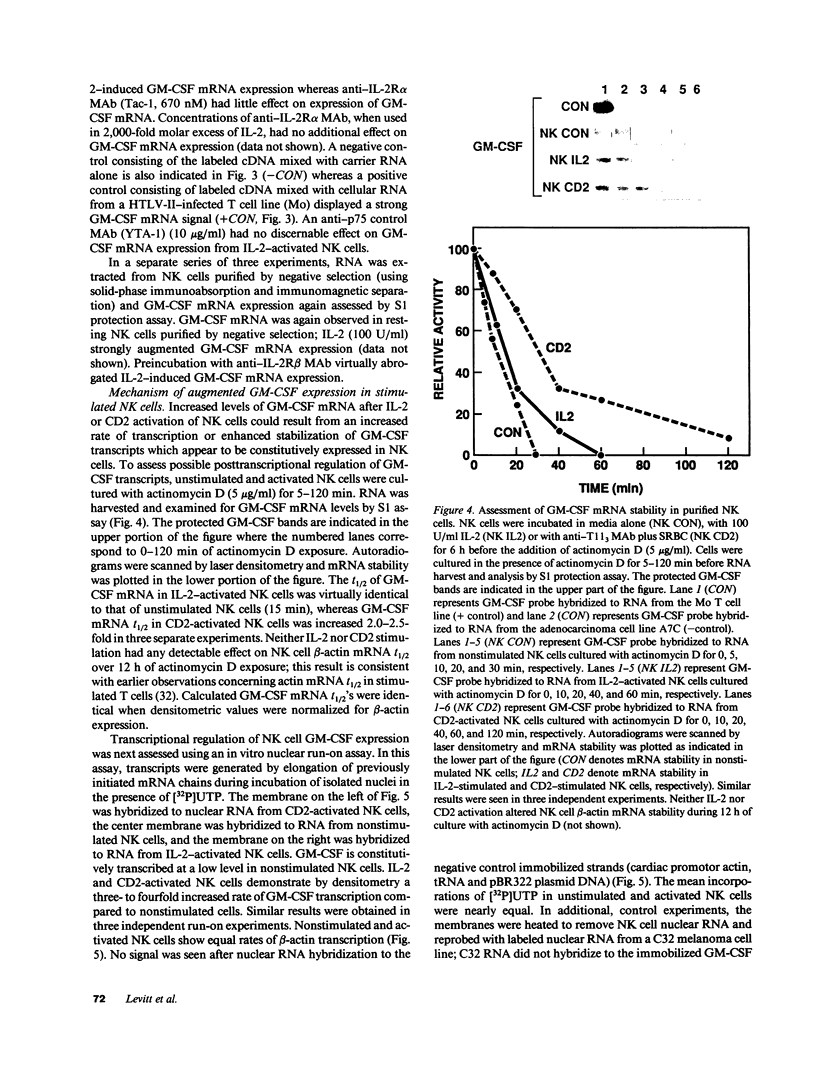
Resting natural killer (NK) cells express the p75 chain of the IL-2 receptor (IL-2R beta) and most NK cells express the CD2 (erythrocyte rosette) receptor. The cell adhesion molecule, LFA-3, is a natural co-ligand for CD2. Tac antigen (IL-2R alpha), a p55 IL-2R subunit, can be expressed after NK activation and may play a role in IL-2-induced NK proliferation. Little is known of the molecular mechanisms underlying cytokine production in NK cells. We investigated the roles of IL-2R alpha, IL-2R beta, and CD2/LFA-3 in the molecular regulation of NK cell granulocyte/macrophage-colony-stimulating factor (GM-CSF) production. Enriched populations of peripheral blood NK cells were separated into CD16-positive and CD16-negative fractions by flow cytometry; positively selected cells were greater than 97% positive for CD16 (the FcIII receptor for IgG which is present on almost all NK cells), less than 1% positive for the T cell antigen CD3, and did not demonstrate rearrangement of the T cell receptor beta chain gene by Southern blot. NK cell supernatants were harvested after 3-4 d of incubation with 0-100 U/ml IL-2, or after incubation with anti-CD2 (T11(3] MAb and sheep red blood cells (SRBC are a homologue for LFA-3). Parallel cell aliquots were harvested at 3-16 h for transcriptional run-on assays, S1 nuclease assays, and actinomycin D mRNA t1/2 determinations. IL-2-activated NK supernatants contained large amounts of GM-CSF (178 +/- 35 pg/ml) by ELISA as did supernatants from CD2-activated NK cells (T11(3) MAb + SRBC: 212 +/- 42) vs. less than 20 pg/ml for NK cells incubated alone or with either SRBC or T11(3) MAb alone. Sepharose-linked anti-CD3 MAb did not induce GM-CSF release from NK cells. By S1 analysis, both IL-2 and CD2 stimulation markedly augmented GM-CSF mRNA expression but with very different latencies of onset. IL-2R beta MAb inhibited greater than 85% of GM-CSF release from IL-2-activated NK cells and markedly suppressed IL-2-induced GM-CSF mRNA expression, whereas IL-2R alpha MAb even at 2,000-fold molar excess of IL-2 had little effect (less than 10%) on either GM-CSF release or mRNA expression. Run-on assays showed that GM-CSF is constitutively transcribed in NK cells and that IL-2 and CD2-activated cells had a three- to fourfold increased rate of GM-CSF transcription compared to nonstimulated cells. The t1/2 of GM-CSF mRNA in IL-2-activated NK cells was identical to that of unstimulated NK cells (15 min), whereas GM-CSF mRNA t1/2 in CD2-activated NK cells was increased 2.5-fold. We conclude that GM-CSF production in NK cells is regulated by both the IL-2Rbeta and the CD2 receptor but not by IL-2Ralpha, that both transcriptional and posttranscriptional signals act together to modulate the level of GM-CSF mRNA in NK cells, and that the molecular mechanisms underlying NK cell GM-CSF production are dependent in part on differential surface receptor activation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anegón I., Cuturi M. C., Trinchieri G., Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988 Feb 1;167(2):452–472. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdach S. E., Levitt L. J. Receptor-specific inhibition of bone marrow erythropoiesis by recombinant DNA-derived interleukin-2. Blood. 1987 May;69(5):1368–1375. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cuturi M. C., Anegón I., Sherman F., Loudon R., Clark S. C., Perussia B., Trinchieri G. Production of hematopoietic colony-stimulating factors by human natural killer cells. J Exp Med. 1989 Feb 1;169(2):569–583. doi: 10.1084/jem.169.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Perussia B., Mangoni L., Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony-inhibiting activity. J Exp Med. 1985 May 1;161(5):1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Greene W. C. The human interleukin-2 receptor: a molecular and biochemical analysis of structure and function. Clin Res. 1987 Sep;35(5):439–450. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Petersson M., Koo G. C., Wigzell H., Kiessling R. In vivo function of natural killer cells as regulators of myeloid precursor cells in the spleen. Eur J Immunol. 1988 Mar;18(3):485–488. doi: 10.1002/eji.1830180326. [DOI] [PubMed] [Google Scholar]
- Herrmann F., Schmidt R. E., Ritz J., Griffin J. D. In vitro regulation of human hematopoiesis by natural killer cells: analysis at a clonal level. Blood. 1987 Jan;69(1):246–254. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kehrl J. H., Dukovich M., Whalen G., Katz P., Fauci A. S., Greene W. C. Novel interleukin 2 (IL-2) receptor appears to mediate IL-2-induced activation of natural killer cells. J Clin Invest. 1988 Jan;81(1):200–205. doi: 10.1172/JCI113295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Tobler A. Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents. Mol Cell Biol. 1988 Aug;8(8):3432–3438. doi: 10.1128/mcb.8.8.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Benike C. J., Phillips J. H., Engleman E. G. Recombinant interleukin 2 enhanced natural killer cell-mediated cytotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu 11 antigens. J Immunol. 1985 Feb;134(2):794–801. [PubMed] [Google Scholar]
- Lanier L. L., Buck D. W., Rhodes L., Ding A., Evans E., Barney C., Phillips J. H. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988 May 1;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Gemmell L., Larson N., Luh J., Arai K., Rennick D. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4360–4364. doi: 10.1073/pnas.82.13.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt L. J., Reyes G. R., Moonka D. K., Bensch K., Miller R. A., Engleman E. G. Human T cell leukemia virus-I-associated T-suppressor cell inhibition of erythropoiesis in a patient with pure red cell aplasia and chronic T gamma-lymphoproliferative disease. J Clin Invest. 1988 Feb;81(2):538–548. doi: 10.1172/JCI113352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt L., Kipps T. J., Engleman E. G., Greenberg P. L. Human bone marrow and peripheral blood T lymphocyte depletion: efficacy and effects of both T cells and monocytes on growth of hematopoietic progenitors. Blood. 1985 Mar;65(3):663–679. [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Matera L., Santoli D., Garbarino G., Pegoraro L., Bellone G., Pagliardi G. Modulation of in vitro myelopoiesis by LGL: different effects on early and late progenitor cells. J Immunol. 1986 Feb 15;136(4):1260–1265. [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nakamura Y., Inamoto T., Sugie K., Masutani H., Shindo T., Tagaya Y., Yamauchi A., Ozawa K., Yodoi J. Mitogenicity and down-regulation of high-affinity interleukin 2 receptor by YTA-1 and YTA-2, monoclonal antibodies that recognize 75-kDa molecules on human large granular lymphocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1318–1322. doi: 10.1073/pnas.86.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T., Miyajima A., Brown N., Otsu K., Abrams J., Saeland S., Caux C., de Waal Malefijt R., de Vries J., Meyerson P. Isolation and characterization of an expressible cDNA encoding human IL-3. Induction of IL-3 mRNA in human T cell clones. J Immunol. 1988 Apr 1;140(7):2288–2295. [PubMed] [Google Scholar]
- Perussia B., Kobayashi M., Rossi M. E., Anegon I., Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987 Feb 1;138(3):765–774. [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V., Ghio R., Nocera A., Leprini A., Perata A., Ferrarini M. Large granular lymphocytes have a promoting activity on human peripheral blood erythroid burst-forming units. Blood. 1985 Feb;65(2):464–472. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Michon J. M., Woronicz J., Schlossman S. F., Reinherz E. L., Ritz J. Enhancement of natural killer function through activation of the T11 E rosette receptor. J Clin Invest. 1987 Jan;79(1):305–308. doi: 10.1172/JCI112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Skettino S., Phillips J., Lanier L., Nagler A., Greenberg P. Selective generation of erythroid burst-promoting activity by recombinant interleukin 2-stimulated human T lymphocytes and natural killer cells. Blood. 1988 Apr;71(4):907–914. [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A., Miller C. W., Norman A. W., Koeffler H. P. 1,25-Dihydroxyvitamin D3 modulates the expression of a lymphokine (granulocyte-macrophage colony-stimulating factor) posttranscriptionally. J Clin Invest. 1988 Jun;81(6):1819–1823. doi: 10.1172/JCI113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Goldman C. K., Bongiovanni K. F., Chan W. C., Winton E. F., Yagita M., Grimm E. A., Waldmann T. A. The p75 peptide is the receptor for interleukin 2 expressed on large granular lymphocytes and is responsible for the interleukin 2 activation of these cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5394–5398. doi: 10.1073/pnas.84.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Birchenall-Sparks M., Kovacs E., Dorman L., Ruscetti F. W. Characterization of interleukin 2 and phorbol myristate acetate augmentation of expression of transfected human interferon-gamma genomic DNA. J Interferon Res. 1988 Aug;8(4):527–538. doi: 10.1089/jir.1988.8.527. [DOI] [PubMed] [Google Scholar]