Abstract
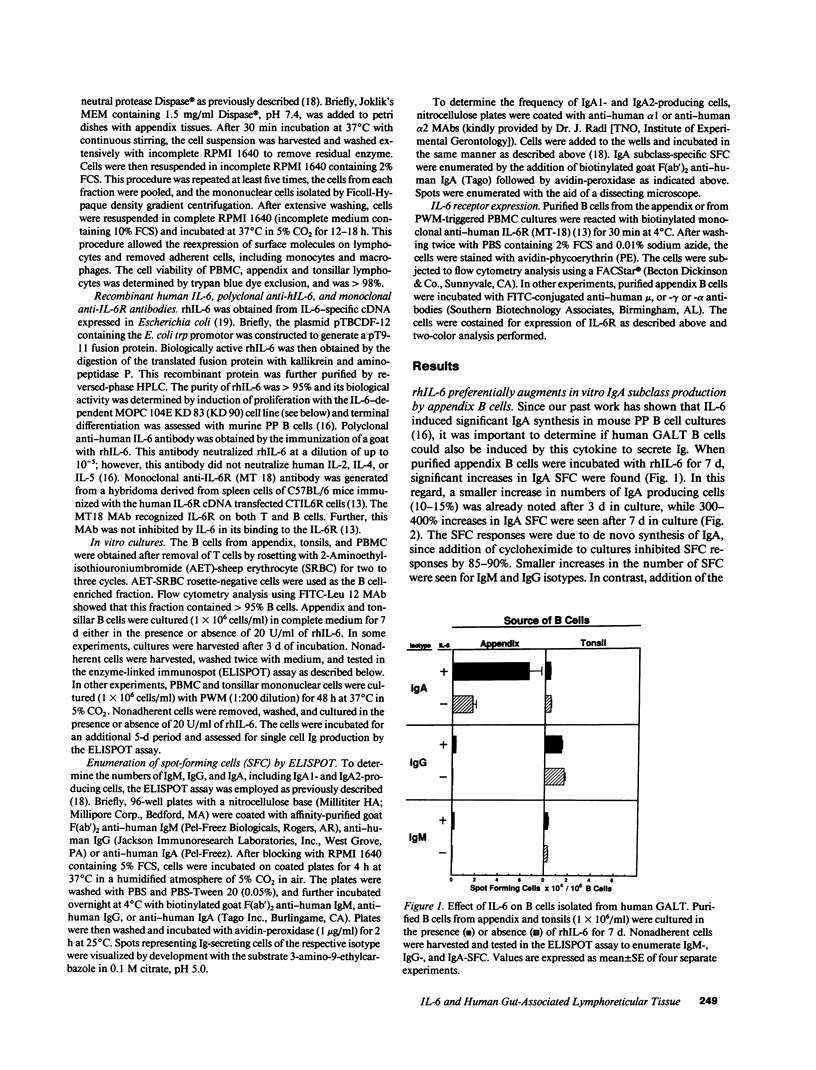
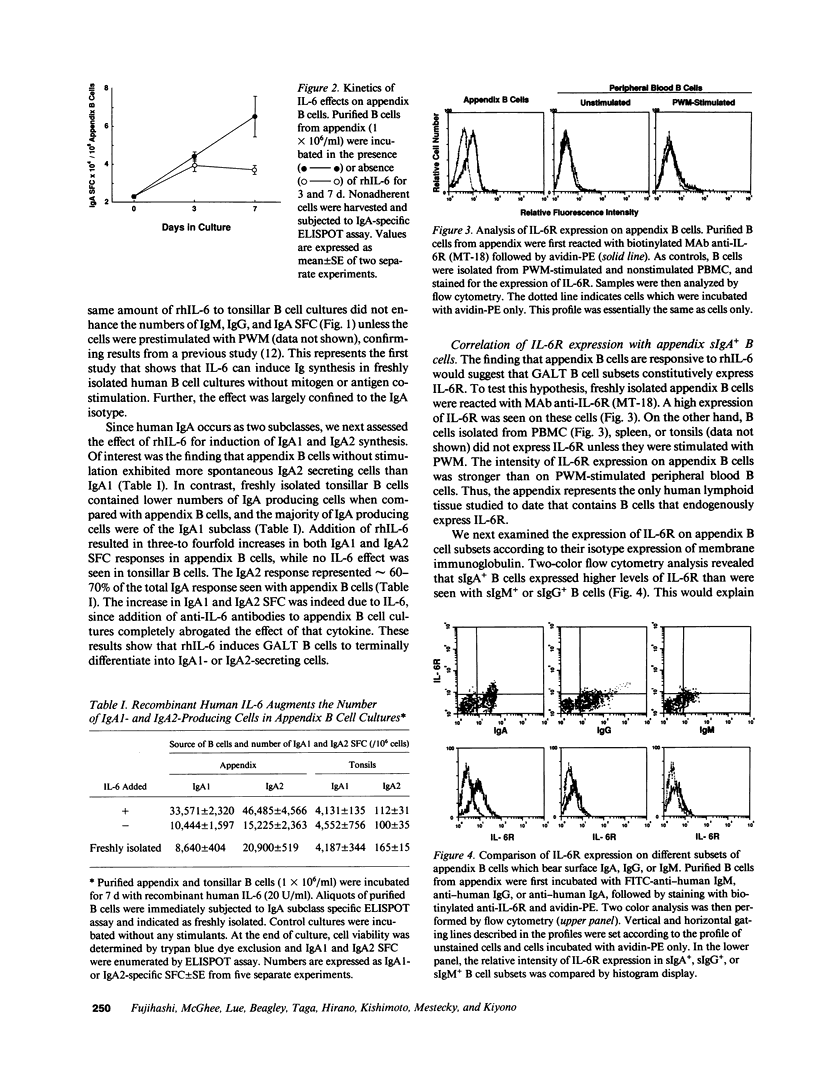
Past studies have shown that freshly isolated human B cells from peripheral blood and tonsils do not express IL-6 receptors (IL-6R); however, mitogen or antigen activation of these B cells induces IL-6R and responsiveness to IL-6. In this study, we have shown that a high proportion of B cells enzymatically dissociated from human appendix, a gut-associated lymphoreticular tissue (GALT), expresses the IL-6R, and that recombinant human IL-6 induces significant increases in the number of Ig-producing cells. The recombinant human IL-6-induced increase in Ig-producing cells is restricted to the IgA isotype. Further, IgA2 is the major subclass; however, significant numbers of IgA1 producing cells are also seen. In contrast, human tonsillar and peripheral blood B cells express low levels of IL-6R, and exogenous IL-6 does not increase numbers of Ig-producing cells. When PBMC or tonsillar cells are stimulated with PWM, the former display an equal distribution of IgA1 and IgA2 secreting cells, while tonsillar B cells are mainly of the IgA1 subclass. The distribution of surface Ig-positive (sIg+) B cells in the appendix B cell population is sIgA+ greater than sIgG+ greater than sIgM+, and the sIgA+ B cells express higher levels of IL-6R when compared with sIgG+ or sIgM+ B cells. These studies show that human appendix contains B cell subsets that constitutively express IL-6R, and that a high proportion of these cells are committed to the IgA isotype. Furthermore, higher numbers of IL-6 responsive IgA2 B cells are present in the human appendix as compared to tonsils or PBMC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beagley K. W., Eldridge J. H., Lee F., Kiyono H., Everson M. P., Koopman W. J., Hirano T., Kishimoto T., McGhee J. R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989 Jun 1;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson E. B., Strober W. Regulation of IgA secretion by T cell clones derived from the human gastrointestinal tract. J Immunol. 1988 Mar 15;140(6):1874–1882. [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P., Rognum T. O. Distribution of immunoglobulin producing cells is different in normal human appendix and colon mucosa. Gut. 1986 Jun;27(6):667–674. doi: 10.1136/gut.27.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E. Functional histology of appendix. Arch Histol Jpn. 1983 Jun;46(3):271–292. doi: 10.1679/aohc.46.271. [DOI] [PubMed] [Google Scholar]
- Crago S. S., Kutteh W. H., Moro I., Allansmith M. R., Radl J., Haaijman J. J., Mestecky J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol. 1984 Jan;132(1):16–18. [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Taga T., Hibi M., Nakano N., Hirano T., Kishimoto T. Characterization of IL-6 receptor expression by monoclonal and polyclonal antibodies. J Immunol. 1989 Nov 1;143(9):2900–2906. [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kett K., Brandtzaeg P., Radl J., Haaijman J. J. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986 May 15;136(10):3631–3635. [PubMed] [Google Scholar]
- Kikutani H., Taga T., Akira S., Kishi H., Miki Y., Saiki O., Yamamura Y., Kishimoto T. Effect of B cell differentiation factor (BCDF) on biosynthesis and secretion of immunoglobulin molecules in human B cell lines. J Immunol. 1985 Feb;134(2):990–995. [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Nakagawa N., Goldstein H., Volkman D. J., Fauci A. S. Demonstration that human B cells respond differently to interleukin 2 and B cell differentiation factor based on their stages of maturation. J Immunol. 1986 Nov 15;137(10):3175–3182. [PubMed] [Google Scholar]
- Ogawa T., Tarkowski A., McGhee M. L., Moldoveanu Z., Mestecky J., Hirsch H. Z., Koopman W. J., Hamada S., McGhee J. R., Kiyono H. Analysis of human IgG and IgA subclass antibody-secreting cells from localized chronic inflammatory tissue. J Immunol. 1989 Feb 15;142(4):1150–1158. [PubMed] [Google Scholar]
- Sehgal P. B., Helfgott D. C., Santhanam U., Tatter S. B., Clarick R. H., Ghrayeb J., May L. T. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988 Jun 1;167(6):1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski J. B., McAnally L. M., Lipsky P. E. IL-2 dependence of the promotion of human B cell differentiation by IL-6 (BSF-2). J Immunol. 1990 Jan 15;144(2):562–569. [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Decock B., Van Snick J., De Ley M., Billiau A. Separation and comparison of two monokines with lymphocyte-activating factor activity: IL-1 beta and hybridoma growth factor (HGF). Identification of leukocyte-derived HGF as IL-6. J Immunol. 1988 Mar 1;140(5):1534–1541. [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


