Abstract
Some murine leukemia viruses (MuLVs), among them Cas-Br-E and ts-1 MuLVs, are neurovirulent, inducing spongiform myeloencephalopathy and hind limb paralysis in susceptible mice. It has been shown that the env gene of these viruses harbors the determinant of neurovirulence. It appears that neuronal loss occurs by an indirect mechanism, since the target motor neurons have not been found to be infected. However, the pathogenesis of the disease remains unclear. Several lymphokines, cytokines, and other cellular effectors have been found to be aberrantly expressed in the brains of infected mice, but whether these are required for the development of the neurodegenerative lesions is not known. In an effort to identify the specific effectors which are indeed required for the initiation and/or development of spongiform myeloencephalopathy, we inoculated gene-deficient (knockout [KO]) mice with ts-1 MuLV. We show here that interleukin-6 (IL-6), inducible nitric oxide synthetase (iNOS), ICE, Fas, Fas ligand (FasL), and TNF-R1 KO mice still develop signs of disease. However, transgenic mice overexpressing Bcl-2 in neurons (NSE/Bcl-2) were largely protected from hind limb paralysis and had less-severe spongiform lesions. These results indicate that motor neuron death occurs in this disease at least in part by a Bcl-2-inhibitable pathway not requiring the ICE, iNOS, Fas/FasL, TNF-R1, and IL-6 gene products.
A few murine leukemia viruses (MuLVs) (e.g., Cas-Br-E and ts-1) are neurovirulent and induce spongiform myeloencephalopathy, leading to hind limb paralysis in susceptible mice (32-34, 91, 106, 109). Results with chimeric MuLV constructed with genes from parental virulent and avirulent MuLVs have established that the envelope (env) gene of these viruses harbors the major determinant of neurovirulence (23, 39, 75, 77, 85, 89, 90, 92, 98) (reviewed in reference 91). In the case of ts-1 MuLV, a single point mutation (Val → Ile) at amino acid position 25 of the coding sequence of the env gene distinguishes it from the parental Moloney MuLV strain from which this ts mutant was derived (98). The important role of the env gene in inducing this disease was also confirmed by expressing the env gene of Cas-Br-E (59) and subsequently of ts-1 (110) MuLV in transgenic (Tg) mice. These env Tg mice developed typical spongiform lesions. ts-1 and Cas-Br-E MuLVs replicate preferentially in microglial cells and to a certain extent in endothelial cells (8, 9, 18, 36, 58, 72, 73, 93), while a third neurovirulent MuLV, PVC-211, replicates mainly in the brain capillary endothelial cells (42, 75). Although replication has been reported by one study to occur in motor neurons (95), this observation has not been confirmed by other studies (36, 58, 72). Therefore, the motor neurons which degenerate in these diseases do not appear to be infected by the virus and are most likely affected indirectly (43, 49, 50, 52, 55).
The spongiform myeloencephalopathy induced by these viruses is most evident in the brain stem and in the lumbar spinal cord. The lesions are characterized by vacuolation, neuronal loss, astrogliosis, activation of microglial cells, and mild demyelination in the absence of an inflammatory reaction (4, 35, 81, 108, 112).
The pathogenesis of this MuLV-induced neurodegenerative disease is not understood. The lesions are very similar to the spongiform lesions induced by the prion agent in mice, but the prion gene itself is dispensable for the development of spongiform lesions, as shown in mice deficient for the prion gene inoculated with neurotropic MuLV (53). In addition, spongiform lesions have been observed in other neurodegenerative diseases, such as Alzheimer's and Parkinson's disease, suggesting that these spongiform neurodegenerative processes may share common effectors.
We have postulated that the MuLV-induced central nervous system (CNS) disease is receptor mediated because of the important role of the env gene in inducing disease (49, 50, 52, 55, 85). Neurotoxins produced by infected microglial cells have also been postulated to represent important mediators of neurovirulence. In particular, MIP-1α, MIP-1β (5, 86), tumor necrosis factor alpha (TNF-α) (5, 14, 78, 86), interleukin-6 (IL-6) (78), Fas/Fas ligand (FasL) (14), and, to a lesser extent, IL-1α (86) and gamma interferon (86) have all been found to be upregulated in the CNS of mice infected with neurovirulent MuLV, although the upregulation of gamma interferon (5, 14) and IL-6 (5, 14, 86) has not been confirmed by other studies. Whether these changes in gene expression are causal in disease development or whether they mainly represent the consequences of the disease process remains unknown. To determine whether some of these molecules are indeed required for the initiation and development of spongiform myeloencephalopathy, we used an alternative genetic approach involving the inoculation with neurovirulent MuLV of mice deficient (knockout [KO]) for a selected gene (TNFR-1, IL-6, inducible nitric oxide synthetase (iNOS), Fas, FasL, or ICE) or mice overexpressing the antiapoptotic gene Bcl-2 in neurons through transgenesis. We report here our results on the inoculation of ts-1 MuLV into these mutant mice.
MATERIALS AND METHODS
Virus.
The ts-1 MuLV (76) was produced in NIH 3T3 cells. Tissue-culture medium containing viruses was filtered through a 0.4-μm-pore-size nitrocellulose filter (Millipore Corporation, Bedford, Mass.) prior to use. The virus was titered at 33°C on NIH 3T3 by using the XC assay, essentially as described previously (51). The virus (∼1 × 105 infectious units/ml) was inoculated intraperitoneally to newborn (<48-h-old) mice. The mice were observed twice a week for signs of disease (abduction reflex, tremulousness, and spastic paralysis of the hind limbs). The infectious virus load in serum and tissues was measured. For the determination of virus load in tissues, spleen, brain, or thymus was homogenized in 2 ml of tissue culture medium and centrifuged at low speed (10,000 × g) and the supernatant was used for an XC assay on NIH 3T3 cells, essentially as described previously (24, 51). Titers were expressed as numbers of PFU per mg of tissue proteins.
Mice.
Mice deficient for the IL-6 (61), Fas (102), or FasL (71, 99) gene were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice deficient for the iNOS (103), ICE (64), or TNF-R1 (87) gene were obtained, respectively, from Foo Y. Liew (Department of Immunology, University of Glasgow), Tara Sesbadri (BASF Bioresearch Corp.), and Tak Mak (Amgen, University of Toronto, Canada). The NSE/Bcl2 (line 71) Tg mice (26, 74) were obtained from Jean-Claude Martinou (Glaxo Institute for Molecular Biology, Geneva, Switzerland). All KO and Tg mice, except the Fas and FasL mutants (already on the C3H background), were bred as heterozygotes on the C3H/HeN (Harland) background for at least five generations before being inoculated. Heterozygote KO males were bred with homozygote female mice to generate hetero- and homozygote littermates. NSE/Bcl-2 Tg mice, heterozygote for the transgene, were bred with normal CH3/HeN mice to generate Tg heterozygote mice and non-Tg littermates, which were used as controls.
Preparation of CNS tissues and immunohistochemistry.
The mice were immersion fixed in 4% paraformaldehyde for 7 days with their skulls open. The brains were then dissected and embedded in paraffin, as described previously (36, 58). The spinal columns were decalcified in 0.5 M EDTA for 20 to 30 days before being embedded in paraffin. Immunocytochemistry with antibodies directed against glial fibrillary acidic protein was performed as previously described (36, 58). Paraffin sections (5 μm) were cut and stained by the conventional hematoxylin and eosin staining method.
Probe preparation and in situ hybridization.
Preparation of 35S-labeled sense and antisense riboprobes specific for the Moloney U3 long terminal repeat (LTR) and glyceraldehyde phosphate dehydrogenase (GAPDH) was carried out as previously described (46). In situ hybridization was carried out as described previously for paraffin-embedded tissues (58).
Image analysis.
Quantification of spongiform lesions was performed on digital images of haematoxylin-and-eosin-stained sections of spinal cord and brain by using Northern Eclipse 6.0 software (Empix Imaging). The sections were scanned and color images were captured by using Zeiss Axiophot supported with Northern Eclipse. For each sagittal brain section, three images were acquired at 10×: one from the anterior pons and two from the central brain stem (see Fig. 3C). For each spinal cord, one section from the lumbar cord was evaluated. The cord area anterior to the central canal was assessed at 10× quantification (2 to 8 images/cord) for spongiform lesions and at 20× quantification (4 to 14 images/cord) for neuron counts.
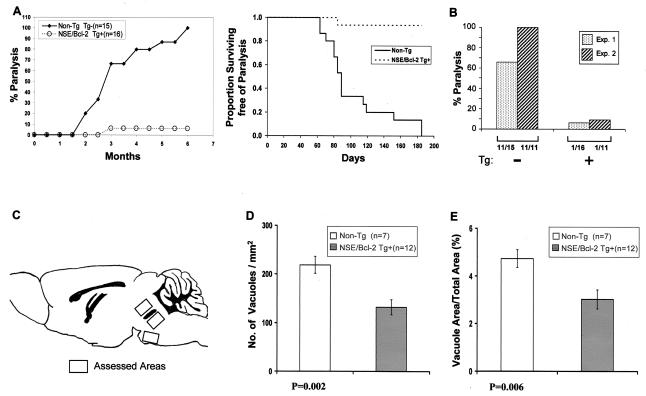
FIG. 3.
Incidence and extent of paralysis in NSE/Bcl-2 Tg mice. (A and B) Newborn Tg and non-Tg control littermates were inoculated with ts-1 MuLV and observed for the development of paralysis. In Experiment 1 (A), the mice were observed for up to 6 months. Data are presented as cumulative incidence (left) and Kaplan-Meir analysis (with log-rank posttest) (right) (P < 0.00001). (B) A second experiment (Experiment 2) was carried out but was terminated at 2.5 months. For comparison, the data from Experiment 1 at 3 months were replotted. The numbers below the groups represent the numbers of paralyzed mice out of the total numbers of mice under experimentation. (C through E) Quantitation of spongiform lesions in the brain stems of ts-1 MuLV-inoculated NSE/Bcl-2 Tg and non-Tg mice. Three separate brain stem areas (C) exhibiting spongiform changes were assessed for the total number of vacuoles per mm2 of tissue surface area (D) and the percentage of tissue area occupied by vacuoles (E). Note that both indices of disease demonstrated a statistically significant reduction in the extent of lesions in Tg mice: (D) non-Tg (n = 7), 218.8 ± 17.6; Tg (n = 12), 131.8 ± 15.4; (E) non-Tg (n = 7), 4.72 ± 0.37; Tg (n = 12), 3.01 ± 0.40 (means ± SEMs).
Spongiform lesions were quantitated in a semiautomated format in sagittal sections of brain stem (three images per mouse; see Fig. 3C) and in the anterior half of the white matter of spinal cord transections (anterior and lateral funiculi to the level of the central canal; 2 to 8 images per mouse). Only vacuoles with diameters of >10 μm and having a round to oblong shape [a shape factor of >0.4, where shape factor = (4 · π · area)÷(perimeter2)] (Northern Eclipse version 6.0 Empix software) were counted. Use of these criteria minimized the inclusion of tissue-processing artifacts (e.g., fine vacuolation caused by solvent extraction of lipids and tissue tears) and of small blood vessels in the analysis. Large blood vessels were manually deleted from the images. Using the thresholding capability of the Northern Eclipse software, the number of vacuoles/mm2, the average diameter per vacuole {obtained by determining the equivalent circle diameter, 2 · [√ (area ÷ π)]}, the average area per vacuole, and the percent of the total area assessed that was occupied by vacuoles were calculated. All quantitation was performed by an investigator who was blinded as to the transgene status of the animals.
For quantitation of neurons, the total neurons in the gray matter of spinal cord were counted manually. At the same time, the same gray matter area was cropped and measured by Northern Eclipse. Then the total number of neurons in each section and the number of total neurons per mm2 were calculated. All quantitation was performed blindly, with the examiner having no information about the transgenic status of the mice.
Statistical analysis.
All data, including the number of vacuoles per mm2, the percentage of the total area occupied by vacuoles, the area per vacuole, the diameter per vacuole, the total number of neurons from each section, and the number of neurons per mm2 from Tg and non-Tg groups were tabulated as means ± standard errors of the means (SEMs). Comparison between Tg and non-Tg groups was performed by using an unpaired two-tailed Student's t test or the Kaplan-Meir analysis with the log-rank posttest. Differences were considered significant if P was <0.05. Except for the Kaplan-Meir analysis, statistical analysis was done using SPSS 7.5 for Windows (SPSS Inc., Chicago, Ill.).
RESULTS
Design of the experiment.
The mutant mice were first put on a genetic background susceptible to ts-1 MuLV. We chose the C3H/HeN background, since previous work has shown this background to be suitable for studies of ts-1 MuLV-induced neurodegenerative disease (76). Except for Fas and FasL mice, all the mutant mice were backcrossed to C3H mice as heterozygotes for at least five generations. At the fifth generation, heterozygote (+/−) and homozygote (−/−) mutant KO mice were crossed together to generate two groups of heterozygote and homozygote mutant mice which were inoculated intraperitoneally as newborns (<48-h-old) with ts-1 MuLV, essentially as described previously (23, 54). The mice were monitored by blinded observers for clinical signs of disease (tremulousness, spasticity, and paralysis). They were sacrificed when they showed clinical signs of paralysis or after 6 months for those showing only minimal or no signs of disease. The majority (75 to 100%) of the inoculated heterozygote or non-Tg control littermates of each mutant (IL-6, iNOS, ICE, TNF-R1, and NSE/Bcl2) strain (n = 63) developed the characteristic hind limb paralysis induced by this virus in normal C3H mice and in other strains (107; Gravel, Kay, and Jolicoeur, unpublished data), as expected. This was also the case for the inoculated heterozygote Fas and FasL mutant mice (n = 24), but the percentage of paralyzed mice was lower, possibly reflecting a slightly different C3H background.
IL-6 appears to be dispensable for the development of spongiform lesions and hind limb paralysis induced by ts-1 MuLV.
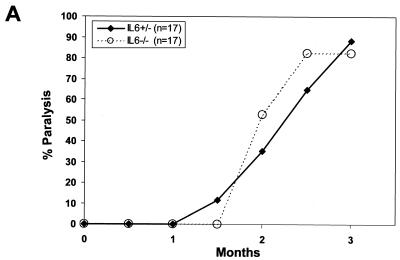
Upregulation of IL-6 in the CNS has been documented in several neurologic diseases, including experimental autoimmune encephalomyelitis (EAE)(84), multiple sclerosis (44), AIDS dementia complex (31), and Alzheimer's disease (10). Moreover, mice deficient in IL-6 are resistant to EAE (27). Overexpression of IL-6 in the CNS of Tg mice was also reported to induce severe neuropathological changes, such as neurodegeneration and astrocytosis and vacuolation (13). In addition, upregulation of IL-6 was found by some (78), but not by all (5, 14, 86), investigators in the CNS of mice developing spongiform disease following inoculation of the neurovirulent MuLVs. In view of this controversy, we used the IL-6-deficient mice to determine the role of this cytokine in the development of MuLV-induced spongiform lesions. Both groups of IL-6+/− and IL-6−/− mice inoculated with ts-1 MuLV developed hind limb paralysis with the same latency (Fig. 1A) and spongiform lesions (Fig. 1B) to the same extent. These results indicate that IL-6 is not necessary for the initiation and development of this disease and that IL-6 deficiency is not protective against the disease.
FIG. 1.
Incidence of paralysis in IL-6 KO mice. (A) Newborn IL-6+/− or IL-6−/− littermate mice were inoculated with ts-1 MuLV and observed for the development of paralysis. (B) Pathological assessment of the CNS of IL-6+/− and IL-6−/− mice. Spongiosis developed comparably in both heterozygote (left) and homozygote (right) mice.
iNOS is not required for the development of spongiform lesions and hind limb paralysis induced by ts-1 MuLV.
Three isoforms of nitric oxide synthetase, endothelial, constitutive, and inducible, are responsible for the production of nitric oxide (79). Nitric oxide appears to have a dual role in the CNS, being both neuroprotective and neurotoxic (15, 57, 94). It may play a role in EAE or in rabies and Borna virus infection, where its levels were reported to be elevated (45). It also appears to mediate human immunodeficiency virus type 1 env (22) and glutamate (21) neurotoxicity. The iNOS isoform is not highly expressed in normal brain (60, 70) but can be activated in response to pathological conditions (47, 96, 101), including multiple sclerosis in humans (6) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease in mice (65). This latter disease was prevented in mice deficient for the iNOS gene (65). Interestingly, the spongiform lesions induced by the neuropathogenic PVC-211 MuLV have been reported to be associated with oxidative damage, reflected by elevated levels of 3-nitrotyrosine immunoreactivity (105) and enhanced expression of iNOS in brain capillary endothelial cells (48a), the major CNS target cells infected by this virus.
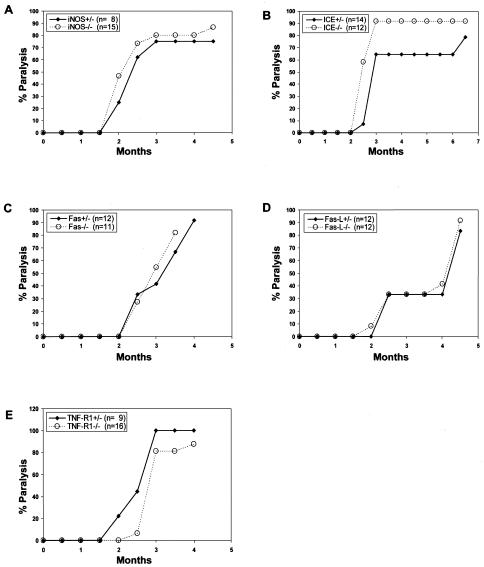
To determine whether the iNOS gene is involved in the development of the spongiform encephalopathy induced by ts-1 MuLV, iNOS+/− and iNOS−/− mutant mice were inoculated with ts-1 MuLV and monitored for signs of disease. In both groups, hind limb paralysis developed after a period of comparative latency (Fig. 2A) and spongiform lesions of comparative severity were formed (data not shown). These results suggest that iNOS is dispensable for the initiation and development of this disease and that iNOS deficiency is not protective against the disease.
FIG. 2.
Incidence of paralysis in iNOS, ICE, Fas, FasL, and TNF-R1 KO mice. Heterozygote and homozygote newborn littermate mice from each strain were inoculated with ts-1 MuLV and observed for the development of paralysis. (A) iNOS; (B) ICE; (C) Fas; (D) FasL; and (E) TNF-R1.
The neuronal cell death induced by ts-1 MuLV is not mediated by ICE, Fas/FasL, or TNF-R1.
The spongiform myeloencephalopathy induced by the neurovirulent MuLVs involves motor neuron loss (4, 12, 82, 88, 97). As in many other neurodegenerative diseases (111), this neuronal cell death has been reported to occur by apoptosis (14, 106). In addition, the expression of TNF-α (5, 14, 78), Fas, and FasL (14) has been reported to be upregulated in mice exhibiting MuLV-induced CNS lesions. Similarly, the levels of IL-1α (86), but not those of IL-1β (5, 14), were found to be elevated in the CNS of these inoculated mice.
The two known receptors for TNF-α, TNF-R1 (p55) and TNF-R2 (p75), are expressed in the CNS, but the function of TNF-R2 in the brain is not well understood (100). TNF-R1 appears to be the major receptor regulating the apoptotic action of TNF-α in various tissues as, in contrast to TNF-R2, it contains an intracellular death domain involved in binding the effectors required for this pathway (7, 16, 68).
Several experimental data point to the involvement of TNF-α in neuronal cell death following various insults (69, 84, 100), including human immunodeficiency virus type 1 infection (104). TNF-α itself, expressed in the CNS of Tg mice, leads to oligodendrocyte apoptosis and myelin vacuolation in the absence of early immune cell infiltration (2), a process similar to the noninflammatory spongiform disease induced by the neurovirulent MuLVs. Interestingly, this TNF-α-induced CNS disease of Tg mice was prevented in mice genetically deficient for the TNF-R1 (p55) gene (2).
Both TNF-R1 and Fas are receptors of the large TNF death receptor family (7, 11, 68). However, the role of the Fas/FasL pathway in apoptosis associated with CNS diseases has not been extensively studied. Enhanced Fas/FasL expression has been reported in the CNS of patients with Alzheimer's-type dementia (80) or multiple sclerosis (25).
IL-1 has been shown to regulate the synthesis of nerve growth factor in glial cells (66). In addition, the processing and release of mature IL-1β is dependent on the activity of caspase-1/ICE (64). Moreover, FasL-induced apoptosis is prevented in caspase-1-deficient mice (62). Caspase-1 is involved in apoptosis in the CNS (112). Mice in which caspase-1 is deficient or inhibited are resistant to hypoxic-ischemic brain damage (38, 67) and to EAE (30) and show a slower disease progression in mouse models of Huntington's disease (83) and amyotrophic lateral sclerosis (29, 63).
In view of previously published results on the perturbation of TNF-α, IL-1, and Fas/FasL in MuLV-induced spongiform myeloencephalopathy and their roles in other CNS diseases, it is conceivable that some of these pathways may be essential for the appearance of MuLV-induced spongiform lesions. Therefore, mice deficient in some of the genes involved in apoptosis were inoculated with ts-1 MuLV.
The majority of ICE −/− (Fig. 2B), Fas−/− (Fig. 2C), FasL−/− (Fig. 2D), and TNF-R1−/− (Fig. 2E) mutant mice and their respective control heterozygote littermates developed hind limb paralysis with comparable latencies and spongiform lesions of comparable severity (data not shown) after inoculation of ts-1 MuLV. The slightly longer latency of paralysis observed in TNF-R1−/− mice and the somewhat higher percentage and slightly shorter latency of paralysis among the ICE−/− mutant mice may or may not be of biological significance. Together, these results suggest that none of these gene products is required for, nor is their absence highly protective against, disease development.
Overexpression of Bcl-2 in neurons protects mice from ts-1 MuLV-induced hind limb paralysis.
The Bcl-2 gene has been shown to protect various cell types, including neurons, from apoptosis induced by very distinct stimuli (3, 20, 26, 28, 56, 74, 113). Overexpression of Bcl-2 in neurons of NSE/Bcl-2 Tg mice has indeed been shown to protect motor neurons from naturally occurring cell death and from experimental ischemia (26, 74). Since motor neurons are the major neural cell population lost in MuLV-induced spongiform disease (4, 12, 82, 88, 97), we used these NSE/Bcl-2 Tg mice (line 71) to determine whether this antiapoptotic molecule would affect the course of the MuLV-induced spongiform encephalopathy.
Groups of NSE/Bcl-2 Tg mice and their non-Tg littermates were inoculated with ts-1 MuLV and monitored for signs of disease. As shown in Fig. 3A, most (15 out of 16) non-Tg C3H littermates developed hind limb paralysis of a severity and after a latency comparable to that of heterozygote C3H mice from the other mutant groups. In contrast, hind limb paralysis was observed in only 1 out of 16 Tg mice, and mild signs of neurological disease (tremulousness and spasticity) were detected in 7 out of 16 Tg mice. In all Tg mice where signs of mild (7 out of 16) or more severe (1 out of 16) clinical disease occurred, the signs developed after a longer period of latency than in non-Tg mice (Fig. 3A). Therefore, frank hind limb paralysis was prevented in the majority (15 out of 16) of NSE/Bcl-2 Tg mice challenged with the virus. A second experiment conducted in a similar group of Tg and non-Tg mice but terminated earlier showed essentially the same results (Fig. 3B). These results clearly established a protective role for Bcl-2 against the clinical manifestations of the disease.
Consistent with this clinical picture, histopathological assessment of the CNS of these mice revealed a reduction in both the extent and the severity of the spongiform lesions (Fig. 3C through E) and of astrogliosis (data not shown) in the brain stem.
To determine whether the milder form of neurodegenerative disease observed in NSE/Bcl-2 Tg mice may be related to increased survival of motor neurons in these Tg mice, we quantitated the motor neurons in the gray matter of the spinal cord. This analysis revealed that these neurons appeared to be more abundant in infected Tg (363 ± 29 neurons/mm2) than in non-Tg (280 ± 45 neurons/mm2) (x ± SEM) spinal cords, suggesting that Tg neurons are protected from death induced by this virus.
Although the expression of the NSE/Bcl-2 transgene was not expected to have an effect on early virus replication in the lymphoid system and on its dissemination in the CNS, we felt it was important to rule out such a detrimental effect of this transgene on virus replication. It has indeed been shown that the kinetics of virus replication in the periphery early after MuLV injection appears to determine the level of CNS infection and consequently the extent and severity of CNS spongiform lesions (17, 19). We therefore measured the extent of ts-1 MuLV infection in the blood, spleen, and CNS of adult and young inoculated NSE/Bcl-2 Tg and non-Tg mice.
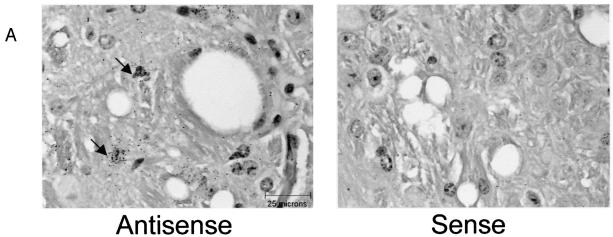
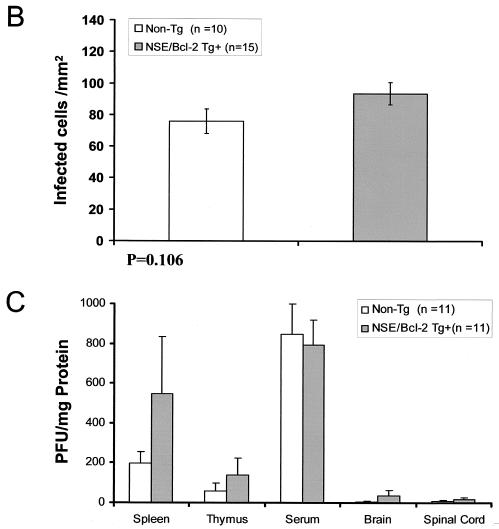
Using in situ hybridization with the U3 LTR-specific probe (Fig. 4A), we found no significant differences in the numbers of infected cells in the CNS of ts-1 MuLV-inoculated NSE/Bcl-2 Tg and non-Tg mice at the time of sacrifice (Fig. 4B). Neither did we observe significant differences in the titers of the virus measured in serum, spleen, thymus, and CNS of 6-day-old NSE/Bcl-2 Tg mice versus the non-Tg littermate controls inoculated as newborns (Fig. 4C). Therefore, these results suggested that the extent of early and late MuLV replication was comparable in Tg and non-Tg mice.
FIG. 4.
Assessment of virus replication in NSE/Bcl-2 Tg mice. (A) In situ hybridization was performed with sense and antisense U3 LTR-specific probes on CNS brain stem sections of ts-1 MuLV-infected adult mice at the time of sacrifice. Note that most infected cells have the appearance of microglial cells, as reported previously. (B) Quantitation of the number of ts-1 MuLV-infected cells from the brain stems of Tg and non-Tg mice. Animals of the same groups as those presented in Fig. 3D were studied. The number of MuLV-infected cells per mm2 of tissues exhibiting spongiform lesions was counted. No statistically significant difference in the number of infected cells was observed between Tg and non-Tg animals: non-Tg (n = 10), 75.8 ± 7.8; Tg (n = 15), 93.6 ± 7.1 (means ± SEMs). (C) Quantitation of infectious ts-1 MuLV in CNS, spleen, thymus, and serum of 6-day-old NSE/Bcl-2 Tg and non-Tg mice infected as newborns (≤36 h). PFUs are given as means + SEMs. No statistically significant differences could be determined between any of the groups of tissues analyzed (P > 0.05, as determined by Student's t test).
DISCUSSION
The cellular and molecular mechanisms by which the spongiform CNS lesions develop and the lower motor neurons are lost after infection of mice by a group of neuropathogenic MuLVs are of considerable interest. The present study was designed to determine whether some specific effectors whose dysregulated expression has previously been found to be associated with the presence of neurodegenerative changes and more specifically of MuLV-induced spongiform lesions were required for the development of MuLV-induced myeloencephalopathy. We took a genetic approach and used several gene-deficient (KO) mouse strains for this analysis. Our data show that mice deficient in the IL-6, iNOS, ICE, Fas, FasL, or TNF-R1 gene were still susceptible to ts-1 MuLV-induced spongiform myeloencephalopathy and hind limb paralysis. These results clearly indicate that these effectors are not required for the initiation or the development and maintenance of the disease. However, in view of possible compensatory gene expression in KO mice, our results cannot necessarily be interpreted as a lack of contribution of these genes to the development of the disease when they are not deleted. The dispensable role of genes representing excellent candidates for involvement in the development of spongiform lesions may reflect their participation in secondary reactive changes which are likely to occur in many neurodegenerative diseases. In fact, our genetic data caution about establishing pathways of neuropathogenesis based exclusively on patterns of gene expression.
Interestingly, constitutive Bcl-2 overexpression in motor neurons of Tg mice was found to have a protective effect against this spongiform ts-1 MuLV-induced disease, leading to an improved clinical outcome (a lower incidence and generally milder form of motor deficits). The brain stem and anterior horn motor neurons have previously been reported to be lost in these MuLV-induced neurodegenerative diseases (4, 12, 82, 88, 97). Motor neurons were targeted to express Bcl-2 in the NSE/Bcl-2 Tg mice (26, 74) used in the present study, and these neurons were previously found to be protected against other insults (axotomy and ischemia) in these Tg mice (26, 74). Therefore, it is likely that the clinical and histological protection observed in ts-1 MuLV-inoculated NSE/Bcl-2 Tg mice reflects protection by Bcl-2 against motor neuron death. In fact, we have documented that loss of anterior spinal cord motor neurons is lessened in these MuLV-inoculated Tg mice compared to that for their non-Tg littermates.
The protection observed in MuLV-infected Tg mice expressing Bcl-2 in motor neurons strongly suggests that these cells are lost by a Bcl-2-inhibitable apoptotic pathway in this neurodegenerative disease. Indeed, Bcl-2 is one of the principal molecules able to protect cells against apoptosis (3, 20, 26, 28, 56, 74, 113). One hypothesis that could explain the beneficial role of Tg Bcl-2 in this disease is downregulation of motor-neuron-endogenous Bcl-2 RNA in the CNS of ts-1 MuLV-infected mice.
The functions of Bcl-2 in cells are complex and not totally elucidated (1, 37). Our data do not establish by which mechanism Bcl-2 protects against neurodegeneration. Since a major site of Bcl-2 action is known to be at the mitochondrial membrane (1, 37), our data suggest that mitochondrial damage may be involved in this MuLV-induced motor neuron disease. Bcl-2 may decrease the generation or increase the scavenging of reactive oxygen species (41, 56) known to be increased in a similar MuLV-induced neurodegenerative disease (105). Alternatively, Bcl-2 may act on other cell organelles. Indeed, Bcl-2 has been shown to protect some cells against apoptosis in ways which are independent of the inhibition of reactive oxygen species (48). Whatever the mechanism, our results with NSE/Bcl-2 mice confirm and extend earlier reports claiming that motor neurons die by apoptosis in ts-1 MuLV-infected mice (14, 106). Our data also indicate that this motor neuron apoptosis pathway does not require signals from Fas or TNF-R1 (two receptors frequently implicated in apoptosis induced in other cells by other stimuli) (7, 11, 68), since mice deficient in Fas, FasL, or TNF-R1 were not protected from development of spongiform lesions. The dispensable role of Fas/FasL in this MuLV-induced neuronal apoptosis is consistent with the protection against apoptosis observed with Bcl-2, since it has been shown that the major Fas/FasL-induced apoptosis pathway cannot be inhibited by Bcl-2, at least in some cells (1). ICE (caspase-1), another effector of some apoptotic stimuli (40), is also dispensable for this motor neuron apoptosis, since ICE-deficient mice were not protected from MuLV-induced neurodegeneration. These ICE (caspase-1) results are also consistent with the dispensable role of Fas/FasL observed, since FasL-induced apoptosis has been shown to be eliminated in caspase-1-deficient mice (62). The lack of protection of MuLV-infected ICE KO mice contrasts with the protection observed against hypoxic-ischemic brain damage in mice whose caspase-1 is deficient or inhibited (38, 67), suggesting that pathways of neuronal cell death induced by ts-1 MuLV and hypoxia or ischemia are not identical.
The identity of the effectors of apoptosis that lead to motor neuron death following infection with neurovirulent MuLVs remains to be determined. An equally important unresolved question is how the env gene of these viruses, which harbors the major determinant of neurovirulence (reviewed in reference 91), triggers this neuronal apoptotic pathway by an indirect mechanism, i.e., without infecting these neurons.
Acknowledgments
This work was supported by a grant to P.J. from the CIHR.
We thank Foo Y. Liew (University of Glasgow) for providing the iNOS gene-deficient mice and Tara Sesbadri and Tariq Ghayurt (formerly of BASF Bioresearch Corp. and now of Abbott) for providing the ICE gene-deficient mice. We thank Ginette Massé, Annie Vallée, Lin Jia, Viorica Lascau, and Eve-Lyne Thivierge for excellent technical assistance, Rita Gingras for preparing the manuscript, and Christian Charbonneau for help with image analysis. We are most grateful to Jean-François Angers and Nadine Ouellette (University of Montreal) for help with statistical analysis.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Akassoglou, K., J. Bauer, G. Kassiotis, M. Pasparakis, H. Lassmann, G. Kollias, and L. Probert. 1998. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am. J. Pathol. 153:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allsopp, T. E., S. Wyatt, H. F. Paterson, and A. M. Davies. 1993. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell 73:295-307. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, J. M., and M. B. Gardner. 1974. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J. Neuropathol. Exp. Neurol. 33:285-307. [DOI] [PubMed] [Google Scholar]
- 5.Askovic, S., C. Favara, F. J. McAtee, and J. L. Portis. 2001. Increased expression of MIP-1α and MIP-1β mRNAs in the brain correlates spatially and temporally with the spongiform neurodegeneration induced by a murine oncornavirus. J. Virol. 75:2665-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagasra, O., F. H. Michaels, Y. M. Zheng, L. E. Bobroski, S. V. Spitsin, Z. F. Fu, R. Tawadros, and H. Koprowski. 1995. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 92:12041-12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, S. J., and E. P. Reddy. 1996. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene 12:1-9. [PubMed] [Google Scholar]
- 8.Baszler, T. V., and J. F. Zachary. 1990. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab. Investig. 63:612-623. [PubMed] [Google Scholar]
- 9.Baszler, T. V., and J. F. Zachary. 1991. Murine retroviral neurovirulence correlates with an enhanced ability of virus to infect selectively, replicate in, and activate resident microglial cells. Am. J. Pathol. 138:655-671. [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, J., S. Strauss, U. Schreiter-Gasser, U. Ganter, P. Schlegel, I. Witt, B. Yolk, and M. Berger. 1991. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer's disease cortices. FEBS Lett. 285:111-114. [DOI] [PubMed] [Google Scholar]
- 11.Bazzoni, F., and B. Beutler. 1996. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 334:1717-1725. [DOI] [PubMed] [Google Scholar]
- 12.Brooks, B. R., J. R. Swarz, and R. T. Johnson. 1980. Spongiform polioencephalomyelopathy caused by a murine retrovirus. I. Pathogenesis of infection in newborn mice. Lab. Investig. 43:480-486. [PubMed] [Google Scholar]
- 13.Campbell, I. L., C. R. Abraham, E. Masliah, P. Kemper, J. D. Inglis, and M. B. M. L. Oldstone. 1993. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA 90:10061-10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe, W., G. Stoica, W. Lynn, and P. K. Wong. 1998. Neurodegeneration induced by MoMuLV-ts1 and increased expression of Fas and TNF-alpha in the central nervous system. Brain Res. 779:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Choi, D. W. 1993. Nitric oxide: foe or friend to the injured brain? Proc. Natl. Acad. Sci. USA 90:9741-9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleveland, J. L., and J. N. Ihle. 1995. Contenders in FasL/TNF death signaling. Cell 81:479-482. [DOI] [PubMed] [Google Scholar]
- 17.Czub, M., S. Czub, F. J. McAtee, and J. L. Portis. 1991. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from central nervous system-specific restriction of virus replication. J. Virol. 65:2539-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czub, M., S. Czub, M. Rappold, S. Mazgareanu, S. Schwender, M. Demuth, A. Hein, and R. Dorries. 1995. Murine leukemia virus-induced neurodegeneration of rats: enhancement of neuropathogenicity correlates with enhanced viral tropism for macrophages, microglia, and brain vascular cells. Virology 214:239-244. [DOI] [PubMed] [Google Scholar]
- 19.Czub, M., F. J. McAtee, and J. L. Portis. 1992. Murine retrovirus-induced spongiform encephalomyelopathy: host and viral factors which determine the length of the incubation period. J. Virol. 66:3298-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, A. M. 1995. The Bcl-2 family of proteins, and the regulation of neuronal survival. Trends Neurosci. 18:355-358. [DOI] [PubMed] [Google Scholar]
- 21.Dawson, V. L., T. M. Dawson, E. D. London, D. S. Bredt, and S. H. Snyder. 1991. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 88:6368-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson, V. L., T. M. Dawson, G. R. Uhl, and S. H. Snyder. 1993. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc. Natl. Acad. Sci. USA 90:3256-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DesGroseillers, L., M. Barrette, and P. Jolicoeur. 1984. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J. Virol. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DesGroseillers, L., E. Rassart, and P. Jolicoeur. 1983. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc. Natl. Acad. Sci. USA 80:4203-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowling, P., G. Shang, S. Raval, J. Menonna, S. Cook, and W. Husar. 1996. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J. Exp. Med. 184:1513-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois-Dauphin, M., H. Frankowski, Y. Tsujimoto, J. Huarte, and J. C. Martinou. 1994. Neonatal motoneurons overexpressing the bcl-2 protooncogene in transgenic mice are protected from axotomy-induced cell death. Proc. Natl. Acad. Sci. USA 91:3309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eugster, H. P., K. Frei, M. Kopf, H. Lassmann, and A. Fontana. 1998. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 28:2178-2187. [DOI] [PubMed] [Google Scholar]
- 28.Farlie, P. G., R. Dringen, S. M. Rees, G. Kannourakis, and O. Bernard. 1995. bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc. Natl. Acad. Sci. USA 92:4397-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedlander, R. M., R. H. Brown, V. Gagliardini, J. Wang, and J. Yuan. 1997. Inhibition of ICE slows ALS in mice. Nature 388:31. [DOI] [PubMed] [Google Scholar]
- 30.Furlan, R., G. Martino, F. Galbiati, P. L. Poliani, S. Smiroldo, A. Bergami, G. Desina, G. Comi, R. Flavell, M. S. Su, and L. Adorini. 1999. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J. Immunol. 163:2403-2409. [PubMed] [Google Scholar]
- 31.Gallo, P., K. Frei, C. Rordorf, J. Lazdins, B. Tavolato, and A. Fontana. 1989. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J. Neuroimmunol. 23:109-116. [DOI] [PubMed] [Google Scholar]
- 32.Gardner, M. B. 1978. Type-C viruses of wild mice: characterization and natural history of amphotropic, ecotropic and xenotropic murine leukemia viruses. Curr. Top. Microbiol. Immunol. 79:215-239. [DOI] [PubMed] [Google Scholar]
- 33.Gardner, M. B. 1985. Retroviral spongiform polioencephalomyelopathy. Rev. Infect. Dis. 7:99-110. [DOI] [PubMed] [Google Scholar]
- 34.Gardner, M. B. 1988. Neurotropic retroviruses of wild mice and macaques. Ann. Neurol. 23(Suppl.):S201-S206. [DOI] [PubMed] [Google Scholar]
- 35.Gardner, M. B., B. E. Henderson, J. E. Officer, R. W. Rongey, J. C. Parker, C. Oliver, J. D. Estes, and R. J. Huebner. 1973. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J. Natl. Cancer Inst. 51:1243-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravel, C., D. G. Kay, and P. Jolicoeur. 1993. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J. Virol. 67:6648-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross, A., J. M. McDonnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 38.Hara, H., R. M. Friedlander, V. Gagliardini, C. Ayata, K. Fink, Z. Huang, M. Shimizu-Sasamata, J. Yuan, and M. A. Moskowitz. 1997. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA 94:2007-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasenkrug, K. J., S. J. Robertson, J. Porti, F. McAtee, J. Nishio, and B. Chesebro. 1996. Two separate envelope regions influence induction of brain disease by a polytropic murine retrovirus (FMCF98). J. Virol. 70:4825-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henkart, P. A. 1996. Ice family proteases: mediators of all apoptotic cell death? Immunity 4:195-201. [DOI] [PubMed] [Google Scholar]
- 41.Hockenbery, D. M., Z. N. Oltvai, X. M. Yin, C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241-251. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman, P. M., E. F. Cimino, D. S. Robbins, R. D. Broadwell, J. M. Powers, and S. K. Ruscetti. 1992. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab. Investig. 67:314-321. [PubMed] [Google Scholar]
- 43.Hoffman, P. M., O. M. Pitts, J. A. Bilello, and E. F. Cimino. 1988. Retrovirus induced motor neuron degeneration. Rev. Neurol. 144:676-679. [PubMed] [Google Scholar]
- 44.Hofman, F. M., D. R. Hinton, K. Johnson, and J. E. Merrill. 1989. Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 170:607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hooper, D. C., S. T. Ohnishi, R. Kean, Y. Numagami, B. Dietzschold, and H. Koprowski. 1995. Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc. Natl. Acad. Sci. USA 92:5312-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang, M., C. Simard, D. G. Kay, and P. Jolicoeur. 1991. The majority of cells infected with the defective murine AIDS virus belong to the B-cell lineage. J. Virol. 65:6562-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iadecola, C., X. Xu, F. Zhang, E. E. el Fakahany, and M. E. Ross. 1995. Marked induction of calcium-independent nitric oxide synthase activity after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 15:52-59. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson, M. D., and M. C. Raff. 1995. Programmed cell death and Bcl-2 protection in very low oxygen. Nature 374:814-816. [DOI] [PubMed] [Google Scholar]
- 48a.Jinno-Oue, A., S. G. Wilt, C. Hanson, N. V. Dugger, P. M. Hoffman, M. Masuda, and S. K. Ruscetti. 2003. Expression of inducible nitric oxide synthase and elevation of tyrosine nitration of a 32-kilodalton cellular protein in brain capillary endothelial cells from rats infected with a neuropathogenic murine leukemia virus. J. Virol. 77:5145-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolicoeur, P. 1990. Retrovirus-induced lower motor neuron disease in mice: a model for amyotrophic lateral sclerosis and human spongiform neurological diseases, p. 52-82. In A. J. Hudson (ed.), Amyotrophic lateral sclerosis. Concepts in pathogenesis and etiology. University of Toronto Press, Toronto, Canada.
- 50.Jolicoeur, P. 1991. Neuronal loss in a lower motor neuron disease induced by a murine retrovirus. Can. J. Neurol. Sci. 18:411-413. [DOI] [PubMed] [Google Scholar]
- 51.Jolicoeur, P., and D. Baltimore. 1975. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J. Virol. 16:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolicoeur, P., C. Gravel, and D. G. Kay. 1992. Pathogenesis of murine spongiform myeloencephalopathy induced by a murine retrovirus, p. 199-224. Humana Press, Totowa, New Jersey.
- 53.Jolicoeur, P., G. Masse, and D. G. Kay. 1996. The prion gene is dispensable for the development of spongiform myeloencephalopathy induced by the neurovirulent Cas-Br-E murine leukemia virus. J. Virol. 70:9031-9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jolicoeur, P., N. Nicolaiew, L. DesGroseillers, and E. Rassart. 1983. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J. Virol. 45:1159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jolicoeur, P., E. Rassart, L. DesGroseillers, Y. Robitaille, Y. Paquette, and D. G. Kay. 1991. Retrovirus-induced motor neuron disease of mice: molecular basis of neurotropism and paralysis. Adv. Neurol. 56:481-493. [PubMed] [Google Scholar]
- 56.Kane, D. J., T. A. Sarafian, R. Anton, H. Hahn, E. B. Gralla, J. S. Valentine, T. Ord, and D. E. Bredesen. 1993. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262:1274-1277. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman, P. L. 1999. Nitric-oxide synthase and neurodegeneration/neuroprotection. Proc. Natl. Acad. Sci. USA 96:9455-9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kay, D., C. Gravel, Y. Robitaille, and P. Jolicoeur. 1991. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc. Natl. Acad. Sci. USA 88:1281-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kay, D. G., C. Gravel, F. Pothier, A. Laperričre, Y. Robitaille, and P. Jolicoeur. 1993. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc. Natl. Acad. Sci. USA 90:4538-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keilhoff, G., B. Seidel, H. Noack, W. Tischmeyer, D. Stanek, and G. Wolf. 1996. Patterns of nitric oxide synthase at the messenger RNA and protein levels during early rat brain development. Neuroscience 75:1193-1201. [DOI] [PubMed] [Google Scholar]
- 61.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Z. R. Kishimoto, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339-342. [DOI] [PubMed] [Google Scholar]
- 62.Kuida, K., J. A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000-2003. [DOI] [PubMed] [Google Scholar]
- 63.Li, M., V. O. Ona, C. Guegan, M. Chen, V. Jackson-Lewis, L. J. Andrews, A. J. Olszewski, P. E. Stieg, J. P. Lee, S. Przedborski, and R. M. Friedlander. 2000. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288:335-339. [DOI] [PubMed] [Google Scholar]
- 64.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, and J. Salfeld. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401-411. [DOI] [PubMed] [Google Scholar]
- 65.Liberatore, G. T., V. Jackson-Lewis, S. Vukosavic, A. S. Mandir, M. Vila, W. G. McAuliffe, V. L. Dawson, T. M. Dawson, and S. Przedborski. 1999. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5:1403-1409. [DOI] [PubMed] [Google Scholar]
- 66.Lindholm, D., R. Heumann, M. Meyer, and H. Thoenen. 1987. Interleukin-1 regulates synthesis of nerve growth factor in non- neuronal cells of rat sciatic nerve. Nature 330:658-659. [DOI] [PubMed] [Google Scholar]
- 67.Liu, X. H., D. Kwon, G. P. Schielke, G. Y. Yang, F. S. Silverstein, and J. D. Barks. 1999. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J. Cereb. Blood Flow Metab. 19:1099-1108. [DOI] [PubMed] [Google Scholar]
- 68.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 69.Loddick, S. A., and N. J. Rothwell. 1999. Mechanisms of tumor necrosis factor alpha action on neurodegeneration: interaction with insulin-like growth factor-1. Proc. Natl. Acad. Sci. USA 96:9449-9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowenstein, C. J., C. S. Glatt, D. S. Bredt, and S. H. Snyder. 1992. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc. Natl. Acad. Sci. USA 89:6711-6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch, D. H., M. L. Watson, M. R. Alderson, P. R. Baum, R. E. Miller, T. Tough, M. Gibson, T. Davis-Smith, C. A. Smith, and K. Hunter. 1994. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1:131-136. [DOI] [PubMed] [Google Scholar]
- 72.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7:365-379. [DOI] [PubMed] [Google Scholar]
- 73.Lynch, W. P., E. Y. Snyder, L. Qualtiere, J. L. Portis, and A. H. Sharpe. 1996. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J. Virol. 70:8896-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinou, J. C., M. Dubois-Dauphin, J. K. Staple, I. Rodriguez, H. M. M. Frankowski, P. Albertini, D. Talabot, S. Catsicas, and C. Pietra. 1994. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017-1030. [DOI] [PubMed] [Google Scholar]
- 75.Masuda, M., P. M. Hoffman, and S. K. Ruscetti. 1993. Viral determinants that control the neuropathogenicity of PVC-211 murine leukemia virus in vivo determine brain capillary endothelial cell tropism of the virus in vitro. J. Virol. 67:4580-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCarter, J. A., J. K. Ball, and J. V. Frei. 1977. Lower limb paralysis induced in mice by a temperature-sensitive mutant of Moloney leukemia virus. J. Natl. Cancer Inst. 59:179-183. [DOI] [PubMed] [Google Scholar]
- 77.Munk, C., J. Lohler, V. Prassolov, U. Just, M. Stockschlader, and C. Stocking. 1997. Amphotropic murine leukemia viruses induce spongiform encephalomyelopathy. Proc. Natl. Acad. Sci. USA 94:5837-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagra, R. M., M. P. Heyes, and C. A. Wiley. 1994. Viral load and its relationship to quinolinic acid, TNF alpha, and IL-6 levels in the CNS of retroviral infected mice. Mol. Chem. Neuropathol. 22:143-160. [DOI] [PubMed] [Google Scholar]
- 79.Nathan, C., and Q. W. Xie. 1994. Nitric oxide synthases: roles, tolls, and controls. Cell 78:915-918. [DOI] [PubMed] [Google Scholar]
- 80.Nishimura, T., H. Akiyama, S. Yonehara, H. Kondo, K. Ikeda, M. Kato, E. Iseki, and K. Kosaka. 1995. Fas antigen expression in brains of patients with Alzheimer-type dementia. Brain Res. 695:137-145. [DOI] [PubMed] [Google Scholar]
- 81.Oldstone, M. B., P. W. Lampert, S. Lee, and F. J. Dixon. 1977. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am. J. Pathol. 88:193-212. [PMC free article] [PubMed] [Google Scholar]
- 82.Oldstone, M. B. A., F. Jensen, F. J. Dixon, and P. W. Lampert. 1980. Pathogenesis of the slow-disease of the central nervous system associated with wild mouse virus. II. Role of virus and host gene products. Virology 107:180-193. [DOI] [PubMed] [Google Scholar]
- 83.Ona, V. O., M. Li, J. P. Vonsattel, L. J. Andrews, S. Q. Khan, W. M. Chung, A. S. Frey, A. S. Menon, X. J. Li, P. E. Stieg, J. Yuan, J. B. Penney, A. B. Young, J. H. Cha, and R. M. Friedlander. 1999. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399:263-267. [DOI] [PubMed] [Google Scholar]
- 84.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161-166. [DOI] [PubMed] [Google Scholar]
- 85.Paquette, Y., Z. Hanna, P. Savard, R. Brosseau, Y. Robitaille, and P. Jolicoeur. 1989. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc. Natl. Acad. Sci. USA 86:3896-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peterson, K. E., S. J. Robertson, J. L. Portis, and B. Chesebro. 2001. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses map to separate regions of the viral envelope gene. J. Virol. 75:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 88.Pitts, O. M., J. M. Powers, J. A. Bilello, and P. M. Hoffman. 1987. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab. Investig. 56:401-408. [PubMed] [Google Scholar]
- 89.Portis, J. L., S. Czub, C. F. Garon, and F. J. McAtee. 1990. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 64:1648-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Portis, J. L., S. Czub, S. Robertson, F. McAtee, and B. Chesebro. 1995. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J. Virol. 69:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Portis, J. L., and W. P. Lynch. 1998. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology 247:127-136. [DOI] [PubMed] [Google Scholar]
- 92.Poulsen, D. J., S. J. Robertson, C. A. Favara, J. L. Portis, and B. W. Chesebro. 1998. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology 248:199-207. [DOI] [PubMed] [Google Scholar]
- 93.Robertson, S. J., K. J. Hasenkrug, B. Chesebro, and J. L. Portis. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J. Virol. 71:5287-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt, H. and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 95.Sharpe, A. H., J. J. Hunter, P. Chassler, and R. Jaenisch. 1990. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature 346:181-183. [DOI] [PubMed] [Google Scholar]
- 96.Simmons, M. L., and S. Murphy. 1992. Induction of nitric oxide synthase in glial cells. J. Neurochem. 59:897-905. [DOI] [PubMed] [Google Scholar]
- 97.Swarz, J. R., B. R. Brooks, and R. T. Johnson. 1981. Spongiform polioencephalomyelopathy caused by a murine retrovirus. II. Ultrastructural localization of virus replication and spongiform changes in the central nervous system. Neuropathol. Appl. Neurobiol. 7:365-380. [DOI] [PubMed] [Google Scholar]
- 98.Szurek, P. F., P. H. Yuen, J. K. Ball, and P. K. Wong. 1990. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J. Virol. 64:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, and T. N. S. Suda. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 100.Venters, H. D., R. Dantzer, and K. W. Kelley. 2000. A new concept in neurodegeneration: TNFα is a silencer of survival signals. Trends Neurosci. 23:175-180. [DOI] [PubMed] [Google Scholar]
- 101.Wallace, M. N., and K. Fredens. 1992. Activated astrocytes of the mouse hippocampus contain high levels of NADPH-diaphorase. Neuroreport 3:953-956. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 103.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 104.Wesselingh, S. L., C. Power, J. D. Glass, W. R. Tyor, J. C. McArthur, J. M. Farber, J. W. Griffin, and D. E. Griffin. 1993. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann. Neurol. 33:576-582. [DOI] [PubMed] [Google Scholar]
- 105.Wilt, S. G., N. V. Dugger, N. D. Hitt, and P. M. Hoffman. 2000. Evidence for oxidative damage in a murine leukemia virus-induced neurodegeneration. J. Neurosci. Res. 62:440-450. [DOI] [PubMed] [Google Scholar]
- 106.Wong, P. K. 1990. Moloney murine leukemia virus temperature-sensitive mutants: a model for retrovirus-induced neurologic disorders. Curr. Top. Microbiol. Immunol. 160:29-60. [DOI] [PubMed] [Google Scholar]
- 107.Wong, P. K., E. Floyd, and P. F. Szurek. 1991. High susceptibility of FVB/N mice to the paralytic disease induced by ts1, a mutant of Moloney murine leukemia virus TB. Virology 180:365-371. [DOI] [PubMed] [Google Scholar]
- 108.Wong, P. K., M. M. Soong, R. MacLeod, G. E. Gallick, and P. H. Yuen. 1983. A group of temperature-sensitive mutants of Moloney leukemia virus which is defective in cleavage of env precursor polypeptide in infected cells also induces hind-limb paralysis in newborn CFW/D mice. Virology 125:513-518. [DOI] [PubMed] [Google Scholar]
- 109.Wong, P. K. Y., and P. H. Yuen. 1992. Molecular basis of neurologic disorders induced by a mutant, ts1, of Moloney murine leukemia virus, p. 161-197. Humana Press. Inc., Totowa, N.J.
- 110.Yu, Y. E., W. Choe, W. Zhang, G. Stoica, and P. K. Wong. 1997. Development of pathological lesions in the central nervous system of transgenic mice expressing the env gene of ts1 Moloney murine leukemia virus in the absence of the viral gag and pol genes and viral replication. J. Neurovirol. 3:274-282. [DOI] [PubMed] [Google Scholar]
- 111.Yuan, J., and B. A. Yankner. 2000. Apoptosis in the nervous system. Nature 407:802-809. [DOI] [PubMed] [Google Scholar]
- 112.Zachary, J. F., C. J. Knupp, and P. K. Wong. 1986. Noninflammatory spongiform polioencephalomyelopathy caused by a neurotropic temperature-sensitive mutant of Moloney murine leukemia virus TB. Am. J. Pathol. 124:457-468. [PMC free article] [PubMed] [Google Scholar]
- 113.Zhong, L. T., T. Sarafian, D. J. Kane, A. C. Charles, S. P. Mah, and R. H. Edwards. 1993. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc. Natl. Acad. Sci. USA 90:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]