Abstract
The Tax protein of human T-cell leukemia virus type 1 (HTLV-1) can form homodimers. Tax dimerization contributes to optimal Tax activity involved in transactivation of the HTLV-1 promoter. The mechanisms used to form specific Tax dimers are poorly understood because the domains that mediate such interactions have not been clearly characterized. Here we have used different approaches (the two-hybrid assay in yeast, the glutathione S-transferase pull-down assay, and the Spot method) to study Tax-Tax interactions. Our results indicate that the integrity of the sequence of Tax, except for the last 16 amino acids (residues 338 to 353), is critical, suggesting that Tax dimerization is dictated more by secondary structure than by primary structure. We were, however, able to delimit a central region involved in Tax self-association that encompasses the residues 127 to 228. This region can be divided into three subdomains of dimerization: DD1 (residues 127 to 146), DD2 (residues 181 to 194), and DD3 (residues 213 to 228). Moreover, the Tax mutants M22 (T130A and L131S) and M29 (K189A and R190S), with amino acid substitutions located in DD1 and DD2, respectively, were found to be impaired in Tax self-association.
Human T-cell leukemia virus type 1 (HTLV-1) Tax protein is a 40-kDa nuclear protein involved in viral transcription regulation (4, 29). Tax does not bind DNA specifically (10, 15) but rather interacts with different members of the activating transcription factor/CRE-binding protein (ATF/CREB) family, including CREB, CREB-2, and CREM (1, 6, 14, 32). These factors are characterized by their basic-leucine zipper C-terminal structures required for DNA binding and protein dimerization. The HTLV-1 promoter carries three conserved 21-bp repeats, called Tax-responsive elements, that contain an imperfect cyclic AMP response element (CRE) recognized by CREB, CREB-2, or CREM (7, 28, 33). Tax first increases the DNA-binding activity of these factors by promoting their basic-leucine zipper domain dimerization (8, 17, 31) and then stabilizes the nucleoprotein complex by direct contacts with nucleotides flanking the CRE site (18, 20, 22). The formation of this complex allows the recruitment of the histone acetyltransferase CREB-binding protein (CBP) or its homologue, p300 (12, 19, 24). The recruitment of CBP/p300 to the HTLV-1 promoter induces local nucleosome modifications by histone acetylation and facilitates stable binding of components of the basal transcription machinery (5, 9, 21).
Tax has been shown to form homodimers (11, 17, 30) that contribute to optimal transcriptional activity from the HTLV-1 promoter (17, 30). The process of Tax self-association, however, still remains largely misunderstood, and domains needed for Tax dimerization have not been fully characterized. Gitlin et al. (11) were the first to demonstrate by chemical cross-linking with glutaraldehyde that Tax can form homodimers. They also suggested that dimer formation was dependent on the cysteines at amino acid residues 153, 174, 212, and 261. On the other hand, Tie et al. (30), by using Tax mutants and cross-linking with BS3, delimited a central region critical for Tax dimerization that extended from residues Thr-123 to Ala-204. Lastly, by using the yeast two-hybrid assay, Jin and Jeang (17) found that the zinc-finger structure localized in the N-terminal part of Tax (residues 22 to 53) was also necessary for its dimerization. If we consider all of these results, it is evident that Tax dimerization cannot be explained by the involvement of a single linear subdomain in Tax. In order to better understand mechanisms of Tax dimerization, we have used three different approaches known to study protein-protein interactions: (i) the two-hybrid assay in yeast, (ii) the glutathione S-transferase (GST) pull-down assay, and (iii) the Spot method. Analyses of Tax mutants indicate that a central region from residues 127 to 228 is involved in Tax self-association. This region can be divided into three subdomains of dimerization: DD1 (residues 127 to 146), DD2 (residues 181 to 194), and DD3 (residues 213 to 228). Moreover, the Tax mutants M22 (T130A and L131S) and M29 (K189A and R190S), with amino acid substitutions located in DD1 and DD2, respectively, were found to be impaired in Tax self-association.
MATERIALS AND METHODS
Two-hybrid assay in yeast.
Tax homodimerization was analyzed by two-hybrid assay in Saccharomyces cerevisiae strain Y190. Strain Y190 possesses the Escherichia coli lacZ gene driven by the GAL4-responsive GAL1 promoter. Wild-type (WT) Tax and mutated Tax were cloned in frame with the GAL4 activation domain of pGAD (Clontech Laboratories, Inc., Palo Alto, Calif.). The Tax mutants M22, M29, M37, and M47 have been described previously (27). The deleted Tax cDNAs were generated by PCR amplification on pSG-Tax, digested by BamHI, and subcloned into pGAD. Yeasts were cotransformed with pAS-Tax and pGAD-Tax by using the lithium acetate method (13), and the β-galactosidase assay, with chlorophenol red-β-d-galactopyranoside (CPRG) as a substrate, was carried out on three independent colonies per transformation as described in the Clontech protocol. The β-galactosidase activity was calculated in Miller units (23).
GST pull-down assay.
WT Tax and mutated Tax cDNAs were cloned in frame into the pGEX plasmid (Amersham Biosciences) that express the Schistosoma japonicum GST. pGEX vectors were transformed into E. coli BL21 to produce GST-Tax fusion proteins, which were purified as described by the manufacturer (Amersham Biosciences). Then, Tax cDNA cloned into pSG was transcribed and translated in the presence of [35S]methionine and [35S]cysteine by using the TNT T7 coupled reticulocyte lysate system of Promega, followed by incubation at 4°C with equal amounts of GST-Tax or GST immobilized on glutathione-Sepharose beads in a buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 250 mM NaCl, and 0.1% Nonidet P-40. After a 2-h incubation, the beads were washed five times with incubation buffer, and the bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by autoradiography.
Spot method.
The cellulose-bound peptides were prepared automatically according to standard spot synthesis protocols (3) by using a spot syntheziser (Abimed GmbH, Langenfeld, Germany). The set of membrane-bound peptides was incubated at 37°C with 8 μg of GST-c-Jun or GST-Tax and peroxidase-conjugated anti-GST (Amersham Biosciences)/ml in a buffer containing 145 mM sucrose, Tris-buffered saline-0.1% Tween 20, and blocking buffer (GenoSys). After 1.5 h of incubation, the membrane was washed twice with Tris-buffered saline-0.2% Tween 20, incubated with enhanced chemiluminescence substrate for the detection of peroxidase (Pierce), and then exposed for 0.5 to 5 min to Hyperfilms-ECL (Amersham Biosciences). The membrane was further treated to remove bound proteins and reused when necessary.
Western blotting.
Proteins were electrophoresed onto an SDS-polyacrylamide gel and blotted to polyvinylidene difluoride membranes (Millipore). The blot was then incubated for 1 h at room temperature with a blocking solution (Tris-buffered saline containing 5% milk) prior to the addition of antiserum. After 2 h at 20°C, the blot was washed four times with Tris-buffered saline-0.2% Tween 20 and incubated for 1 h with goat anti-mouse immunoglobulin-peroxidase conjugate (Beckman Coulter). After three washes, the membrane was incubated with enhanced chemiluminescence substrate for the detection of peroxidase (Pierce). The membrane was then exposed for 0.5 to 5 min to Hyperfilms-ECL (Amersham Biosciences). The GAL4 activation domain (AD) monoclonal antibodies were purchased from Clontech.
RESULTS AND DISCUSSION
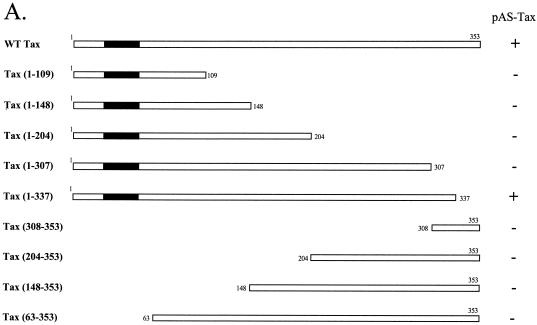
To map the domains in Tax that were required for its homodimerization, we first tested various N- and C-terminal truncations of Tax by the yeast two-hybrid assay. Each of the Tax mutants was fused to the GAL4 activation domain (pGAD-Tax) and tested in yeast for its interaction with WT Tax fused to the GAL4 DNA-binding domain (pAS-Tax). When the last 16 C-terminal amino acids of Tax were deleted [pGAD-Tax(1-337)], yeasts cotransformed with this mutant and WT Tax showed β-galactosidase activity equivalent to that obtained with yeasts cotransformed with pGAD-Tax and pAS-Tax (Fig. 1). On the other hand, with further C-terminal deletions of Tax, no β-galactosidase activity was detected. In the same way, all of the Tax mutants with the N-terminal cysteine-rich zinc-binding domain truncated were unable to interact with WT Tax (Fig. 1A). As shown in Fig. 1B, absence of β-galactosidase activity was not due to an instability of truncated Tax in yeast. Thus, by this approach, we obtained results that failed to reveal a single linear region involved in Tax self-association. In addition, these results suggest that Tax dimerization in vivo is dictated more by secondary structure than by primary structure and that the C- and N-terminal regions are probably necessary to configure Tax into a proper secondary conformation.
FIG. 1.
Study of Tax self-association by yeast two-hybrid assay. (A) Schematic representation of Tax proteins fused to the GAL4 activation domain: the cysteine-rich zinc-binding domain is represented by a black box. The Y190 yeast cells were transformed with either WT Tax or truncations of Tax fused to the activation domain of GAL4 and the plasmid pAS-Tax (WT Tax fused to the GAL4-DNA-binding domain). The β-galactosidase assay with CPRG as substrate was carried out on three independent colonies per transformation. Symbols: +, β-galactosidase activity corresponding to the level of interaction obtained with WT Tax; −, no stimulation of β-galactosidase activity. (B) Stability of the different mutant Tax proteins fused to the GAL4 activation domain in S. cerevisiae. Yeast extracts were analyzed by SDS-PAGE and Western blotting with GAL4 AD monoclonal antibodies. Molecular size markers (in kilodaltons) are shown on the left.
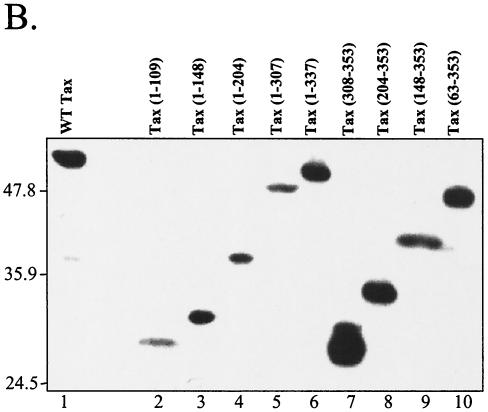
Since Tax secondary structure is a limiting factor to study the protein homodimerization in vivo, the interactions between the truncated Tax mutants with WT Tax were then studied by in vitro assay. Mutated Tax proteins were cloned in frame with GST, expressed in E. coli, immobilized on glutathione-Sepharose beads, and incubated with Tax translated in the presence of [35S]methionine and [35S]cysteine. As shown in the Fig. 2A, GST-Tax but not GST allowed the efficient capture of WT Tax on glutathione-Sepharose (for instance, compare lane 2 with lane 3). Moreover, several subdomains of Tax fused to GST were able to capture WT Tax. The first one corresponds to the N-terminal region (Fig. 3) encompassing the cysteine-rich zinc-binding domain (amino acids 22 to 53 [25]). Indeed, Tax(1-66), Tax(1-109), and Tax(1-148) (Fig. 2A, lanes 4, 5, and 6) interacted with WT Tax, but Tax(65-114) (Fig. 2A, lane 14) was no longer able to bind to Tax (Fig. 3). This observation confirms previous results suggesting that the zinc-finger domain was necessary for Tax dimerization (17). A second subdomain involved in Tax-Tax interactions is comprised in the central region of Tax (Fig. 3). Indeed, the truncated mutant Tax(114-204) strongly interacted with WT Tax (Fig. 2A, lanes 7 and 11). Lastly, whereas Tax(308-353) did not capture radiolabeled Tax (Fig. 2A, lane 21), Tax(204-353) did so (Fig. 2A, lane 20), suggesting that a last subdomain of interaction is present between residues 204 and 308 (Fig. 3). Taken together, these data obtained by GST pull-down assay reveal that different subdomains of Tax are necessary for its homodimerization.
FIG. 2.
Study of Tax-Tax interactions by GST-pull down assay. (A) In vitro-translated WT Tax in the presence of [35S]methionine and [35S]cysteine (lanes 1, 8, and 15) was incubated with equal amounts of purified GST (lanes 2, 9, and 16), GST-WT (lanes 3, 10, and 17), or GST-truncated Tax (lanes 4, 5, 6, 7, 11, 12, 13, 14, 18, 19, 20, and 21) immobilized on glutathione-Sepharose beads. The upper panels show Western blot analyses performed to detect the amounts of protein for the different mutant Tax proteins fused to the GST by using peroxidase-conjugated anti-GST. Molecular size markers are shown on the left in kilodaltons. In the lower panels, the bound 35S-labeled Tax was revealed, following incubation, by autoradiography after SDS-PAGE and then quantified by scanning with Scion Image. The results are reported in calibrated units (CU) under each lane. (B) Coomassie blue-stained SDS-PAGE gels show the purity rate of the GST-fused proteins.
FIG. 3.
Results of the study of Tax self-association obtained by GST pull-down assay. Schematic representation of Tax proteins fused to GST. The cysteine-rich zinc-binding domain is represented by a black box. The 35S-radiolabeled Tax was incubated with equal amounts of GST, GST-WT, or truncated Tax. Each mutant was analyzed three times, with similar results. Symbols: +, signal intensity corresponding the interaction between 35S-labeled Tax and GST-WT Tax; −, interaction between 35S-labeled Tax and GST.
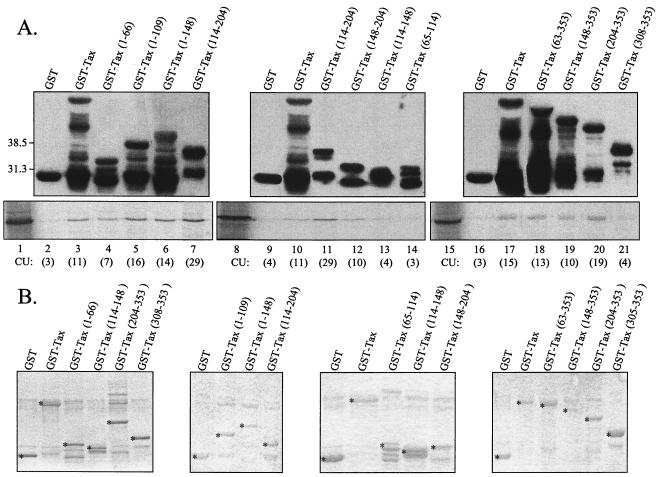
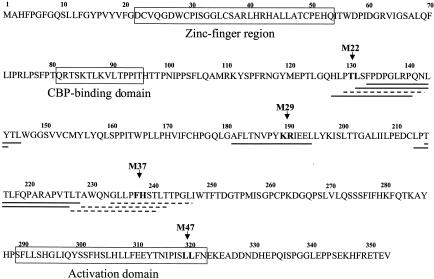
To map more precisely the subdomains involved in Tax dimerization, the complete amino acid sequence of Tax was synthesized on a cellulose membrane support as a set of 171 overlapping peptides of 14 amino acids frameshifted by two residues. In this method, the peptides remain attached to the cellulose membrane used for the synthesis. Their capacity to specifically bind Tax was analyzed by incubating the membrane with purified GST-Tax, and the interaction was visualized by peroxidase-conjugated anti-GST (Fig. 4B). Binding of GST-Tax to Tax peptides essentially focused on three distinct regions, including (i) peptides 63 to 66, with a stronger signal for the peptide 65 (131-LSFPDPGLRPQNLY-144); (ii) peptide 90 (181-AFLTNVPYKRIEEL-194), and (iii) peptides 106 and 107, with a stronger interaction with peptide 106 (213-LPTTLFQPARAPVT-226). A faint but reproducible interaction with the peptides 113, 114, and 116 was also observed (Fig. 4B). When the membrane was incubated with GST-c-Jun as negative control, no interaction was detected (Fig. 4A). The data obtained by the Spot technique (Fig. 5) confirm that three subdomains of Tax are involved in Tax self-association.
FIG. 4.
Identification by the Spot method of Tax peptides recognized by GST-Tax. The set of membrane-bound peptides was incubated with GST-c-Jun (A) or GST-Tax (B). The binding was revealed by peroxidase-conjugated anti-GST.
FIG. 5.
Amino acid sequence of Tax and mapping of the peptides recognized by Tax by the Spot method. The peptides recognized by GST-Tax are underlined. The dotted lines correspond to weak interactions between the peptides and GST-Tax.
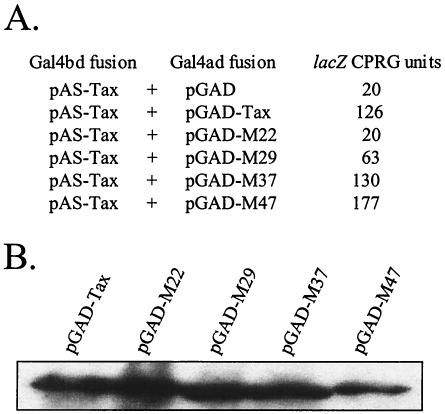
Having determined that different subdomains could be involved in Tax homodimerization, we analyzed a subset of Tax mutants (M22, M29, and M37), previously generated by Smith and Greene (27), in their capacity to homodimerize. As shown in Fig. 5, each mutation is located in one of the three characterized subdomains. Mutants M22 (T130A and L131S) and M37 (F237A and H238S) are reduced, whereas mutant M29 (K189A and R190S) is inhibited, in their capacities to transactivate the HTLV-1 promoter (27). As a negative control, we tested M47 (L319R and L320S), that is also inhibited in transactivation of the HTLV-1 promoter. Each mutant was fused to the GAL4 activation domain and tested in yeast for interaction with WT Tax (Fig. 6). Although M37 and M47 were able to interact with WT Tax, M29 bound WT Tax modestly. Moreover, no β-galactosidase activity was detected in yeast transformed with M22 and WT Tax. The interactions were also analyzed by in vitro assay. By this approach, WT Tax interacted with every tested Tax mutant, but the interactions with M22 and M29 were weaker than those with M37 and M47 (Fig. 7). Taken together, these results show that the mutations in M22 and M29 are effectively located in subdomains important for Tax self-association. They also confirm the data obtained by BS3 cross-linking where M22 yielded a low level of cross-linker dimer (30). However, it is the first time that M29 is described to be impaired in Tax-self association. On the other hand, we detected no difference between M37/Tax and Tax/Tax interactions by the approaches we used. This result is not totally unexpected since M37 is located in a region of Tax that is weakly recognized by the Spot technique (see Fig. 5). Although it would be of interest to test Tax mutations located upstream from M37, between amino acids 213 and 228, no such mutants are currently available (26, 27).
FIG. 6.
Analysis of interactions of mutant Tax proteins in yeast. (A) The Y190 yeast cells were transformed with pAS-Tax and mutant Tax proteins fused to the GAL4 activation domain (pGAD-M22, -M29, -M37, or -M47). The β-galactosidase assay with CPRG as substrate was carried out on three independent colonies per transformation. The β-galactosidase activity was calculated in Miller units. (B) Stability of the different mutant Tax proteins fused to the GAL4 activation domain in S. cerevisiae. Yeast extracts were analyzed by SDS-PAGE and Western blotting with GAL4 AD monoclonal antibodies.
FIG. 7.
Analysis of interactions of mutant Tax proteins in vitro. Radiolabeled WT Tax (lanes 1, 7, 13, and 19), M22 (lane 4), M29 (lane 10), M37 (lane 16), or M47 (lane 22) was incubated with GST (lanes 2, 5, 8, 11, 14, 17, 20, and 23) or GST-WT Tax (lanes 3, 6, 9, 12, 15, 18, 21, and 24). The bound 35S-labeled Tax was analyzed as described in the legend of Fig. 2 and quantified by scanning. The results are reported in calibrated units (CU) under each lane.
In the present study we show that a central region, from residues 127 to 228, is involved in Tax homodimerization. This region can be divided into three subdomains: DD1 (residues 127 to 146), DD2 (residues 181 to 194), and DD3 (residues 213 to 228). Interestingly, the mutated residues of the Tax mutants M22 and M29, which we found impaired in Tax self-association, are included in the subdomains DD1 and DD2, respectively. Moreover, the three domains are located in the central region of Tax, for which no particular function has yet been determined. In addition, the results presented here not only provide additional detailed information on Tax-Tax interactions, but they also corroborate those published previously (30). However, we cannot totally exclude that one or the other of the different subdomains characterized by the in vitro approaches are not surface exposed in the native structure and then not involved in Tax-Tax interactions in vivo. Only determination of the crystal structure of Tax will definitively answer this question.
The data obtained with the GST pull-down assay suggest that the N-terminal zinc-finger domain could be also involved in Tax dimerization. This observation is confirmed by the results obtained in vivo with the yeast two-hybrid assay, for which the mutant Tax(63-353) is no longer able to interact with WT Tax, as previously reported by Jin and Jeang (17). However, the exact function of the zinc-finger domain is not clear at present. It could be directly involved in Tax-Tax interactions or only necessary to configure Tax into a proper secondary conformation for the interaction with cellular partners (2). Such a structure has also been described to be involved in the multimerization of human immunodeficiency virus type 1 integrase (34), which is a 32-kDa protein comprised of three structurally and functionally distinct domains: the N-terminal domain containing a pair of His and Cys residues involved in zinc binding, the central core domain, and the C-terminal domain. Tetramerization of human immunodeficiency virus type 1 integrase requires only the core and C-terminal domains (16). However, when zinc is bound, the full-length protein forms tetramers at a lower concentration than when in its absence (34), suggesting that one role of the N-terminal domain of the integrase may be to facilitate assembly of the active multimeric form of the enzyme. The zinc finger of Tax may play a similar role by favoring proper folding of the central region of Tax and then its homodimerization. In conclusion, taken together, our results suggest that interactions between different domains of Tax is needed for functional dimerization.
Acknowledgments
This study was supported by institutional grants from the Centre National de la Recherche Scientifique (CNRS) and the Université Montpellier I (UM I). J.B. is a fellow of the Centre National de la Recherche Scientifique (Bourse Docteur Ingénieur du CNRS).
We thank W. C. Greene for Tax mutants M22, M29, M37, and M47.
REFERENCES
- 1.Adya, N., and C.-Z. Giam. 1995. Distinct regions in human T-cell lymphotropic virus type 1 Tax mediate interactions with activator protein CREB and basal transcription factors. J. Virol. 69:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun, A., Y. Zhou, C.-M. Wong, H.-K. Kung, K.-T. Jeang, and D.-Y. Jin. 2000. Coiled-coil motif as a structural basis for the interaction of HTLV type I Tax with cellular cofactors. AIDS Res. Hum. Retrovir. 16:1689-1694. [DOI] [PubMed] [Google Scholar]
- 3.Frank, R. 1992. Spot-Sybthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48:9217-9232. [Google Scholar]
- 4.Franklin, A. A., and J. K. Nyborg. 1995. Mechanisms of Tax regulation of human T-cell leukemia virus type I gene expression. J. Biomed. Sci. 2:17-29. [DOI] [PubMed] [Google Scholar]
- 5.Gachon, F., C. Devaux, and J. M. Mesnard. 2002. Activation of HTLV-1 transcription in the presence of Tax is independent of the acetylation of CREB-2 (ATF-4). Virology 299:271-278. [DOI] [PubMed] [Google Scholar]
- 6.Gachon, F., A. Péléraux, S. Thébault, J. Dick, I. Lemasson, C. Devaux, and J. M. Mesnard. 1998. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J. Virol. 72:8332-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gachon, F., S. Thebault, A. Peleraux, C. Devaux, and J. M. Mesnard. 2000. Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4). Mol. Cell. Biol. 20:3470-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudray, G., F. Gachon, J. Basbous, M. Biard-Piechaczyk, C. Devaux, and J. M. Mesnard. 2002. The complementary strand of HTLV-1 RNA genome encodes a bZIP transcription factor that down-regulates the viral transcription. J. Virol. 76:12813-12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georges, S. A., W. L. Kraus, K. Luger, J. K. Nyborg, and P. J. Laybourn. 2002. p300-mediated Tax transactivation from recombinant chromatin: histone tail deletion mimics coactivator function. Mol. Cell. Biol. 22:127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giam, C.-Z., and Y.-L. Xu. 1989. HTLV-1 Tax gene product activates transcription via preexisting cellular factors and cAMP responsive element. J. Biol. Chem. 264:15236-15241. [PubMed] [Google Scholar]
- 11.Gitlin, S. D., P. F. Lindholm, S. J. Marriott, and J. N. Brady. 1991. Transdominant human T-cell lymphotropic virus type I TAX1 mutant that fails to localize to the nucleus. J. Virol. 65:2612-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrod, R., Y. Tang, C. Nicot, H. S. Lu, A. Vassilev, Y. Nakatani, and C.-Z. Giam. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 18:5052-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, H., Y. Fukada, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeang, K.-T., R. Chiu, E. Santos, and S.-J. Kim. 1991. Induction of the HTLV-1 LTR by Jun occurs through the Tax-responsive 21-bp elements. Virology 181:218-227. [DOI] [PubMed] [Google Scholar]
- 15.Jeang, K. T., I. Boros, J. Brady, M. Radanovich, and G. Khoury. 1988. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J. Virol. 62:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins, T., A. Engelman, R. Ghirlando, and R. Craigie. 1996. A soluble active mutant of HIV-1 integrase. J. Biol. Chem. 271:7712-7718. [DOI] [PubMed] [Google Scholar]
- 17.Jin, D.-Y., and K.-T. Jeang. 1997. HTLV-1 Tax self-association in optimal transactivation function. Nucleic Acids Res. 25:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimzey, A. L., and W. S. Dynan. 1998. Specific regions of contact between human T-cell leukemia virus type I Tax protein and DNA identified by photocross-linking. J. Biol. Chem. 273:13768-13775. [DOI] [PubMed] [Google Scholar]
- 19.Kwok, R. P. S., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H.-M. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the coactivator CBP. Nature 370:223-226. [DOI] [PubMed] [Google Scholar]
- 20.Lenzmeier, B. A., H. A. Giebler, and J. K. Nyborg. 1998. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol. Cell. Biol. 18:721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, H., C. A. Pise-Masison, T. M. Fletcher, R. L. Schiltz, A. K. Nagaich, M. Radonovich, G. Hager, P. A. Cole, and J. N. Brady. 2002. Acetylation of nucleosomal histones by p300 facilitates transcription from Tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol. Cell. Biol. 22:4450-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundblad, J. R., R. P. S. Kwok, M. E. Laurance, M. S. Huang, J. P. Richards, R. G. Brennan, and R. H. Goodman. 1998. The human T-cell leukemia virus-1 transcriptional activator Tax enhances cAMP-responsive element-binding protein (CREB) binding activity through interactions with the DNA minor groove. J. Biol. Chem. 273:19251-19259. [DOI] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Scoggin, K. E. S., A. Ulloa, and J. K. Nyborg. 2001. The oncoprotein Tax binds the SRC-1-interacting domain of CBP/p300 to mediate transcriptional activation. Mol. Cell. Biol. 21:5520-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semmes, O. J., and K.-T. Jeang. 1992. HTLV-1 Tax is a zinc-binding protein: role of zinc in Tax structure and function. Virology 188:754-764. [DOI] [PubMed] [Google Scholar]
- 26.Semmes, O. J., and K.-T. Jeang. 1992. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J. Virol. 66:7183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-1 tax transactivator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, T., J. I. Fujisawa, M. Toita, and M. Yoshida. 1993. The transactivator Tax of human T-cell leukemia virus type I (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. USA 90:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thébault, S., F. Gachon, G. Gaudray, and J. M. Mesnard. 2001. Regulation of human T-cell leukemia virus type I genome transcription by the viral Tax protein. Rec. Res. Dev. Virol. 3:151-164. [Google Scholar]
- 30.Tie, T., N. Adya, W. C. Greene, and C.-Z. Giam. 1996. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J. Virol. 70:8368-8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner, S., and M. R. Green. 1993. HTLV-1 Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science 262:395-399. [DOI] [PubMed] [Google Scholar]
- 32.Yin, M.-J., E. J. Paulssen, J.-S. Seeler, and R. B. Gaynor. 1995. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I Tax and CREB. J. Virol. 69:3420-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, L. J., and C.-Z. Giam. 1992. Human T-cell lymphotropic virus type I (HTLV-1) transcriptional activator, Tax, enhances CREB binding to HTLV-1 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 89:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng, R., T. Jenkins, and R. Craigie. 1996. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc. Natl. Acad. Sci. USA 93:13659-13664. [DOI] [PMC free article] [PubMed] [Google Scholar]