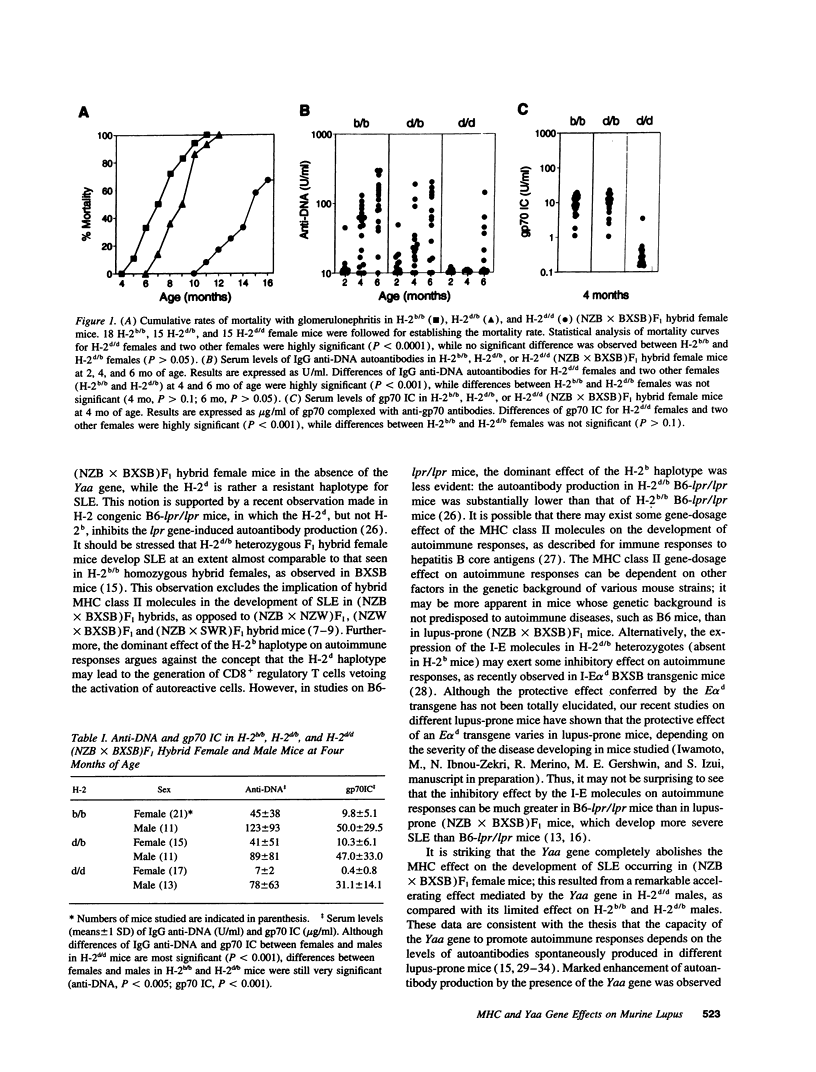
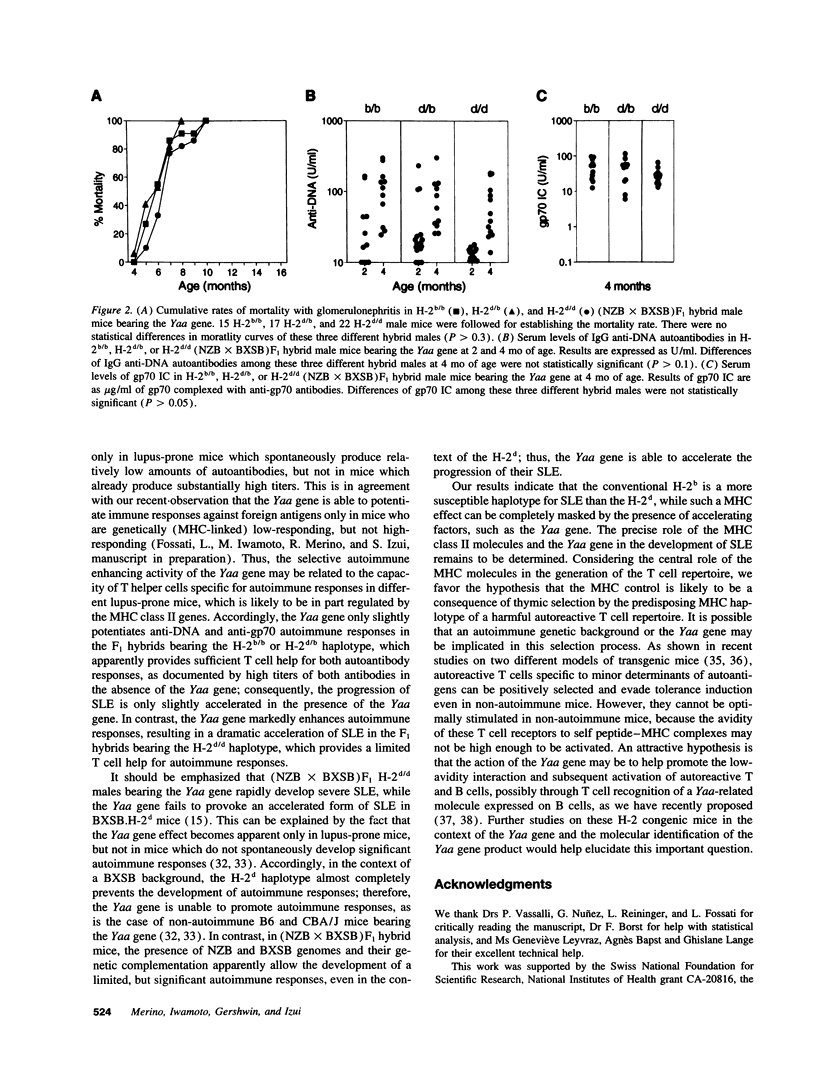
Abstract
To investigate the specific contribution of select MHC class II genes on the development of murine lupus, H-2 congenic (NZB x BXSB)F1 hybrid mice bearing either H-2b/b, H-2d/b, or H-2d/d haplotypes were generated. We compared the clinical development (autoantibody production and glomerulonephritis) of systemic lupus erythematosus (SLE) in these three F1 hybrids in the presence or absence of the mutant gene, Yaa (Y chromosome-linked autoimmune acceleration), which normally accelerates the progression of murine SLE. (NZB x BXSB)F1 hybrid female mice bearing either the H-2b/b or H-2d/b haplotype developed a rapid course of severe SLE, while the appearance of disease was markedly delayed in H-2d/d hybrid females. However, in the presence of the Yaa gene, H-2d/d F1 males developed SLE as severe as H-2b/b and H-2d/b F1 males. These data indicate that (a) the conventional H-2b is a haplotype leading to susceptibility for murine SLE, while H-2d is a relatively resistant haplotype; (b) the H-2b haplotype exhibits a dominant effect on autoimmune responses, similar to the classical MHC-linked Ir gene effect; and (c) most strikingly, the Yaa gene totally abrogates the MHC effect on murine lupus in (NZB x BXSB)F1 hybrid mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. Regions of allelic hypervariability in the murine A alpha immune response gene. Cell. 1983 Aug;34(1):169–177. doi: 10.1016/0092-8674(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Bocchieri M. H., Cooke A., Smith J. B., Weigert M., Riblet R. J. Independent segregation of NZB immune abnormalities in NZB x C58 recombinant inbred mice. Eur J Immunol. 1982 Apr;12(4):349–354. doi: 10.1002/eji.1830120417. [DOI] [PubMed] [Google Scholar]
- Breslow N. Covariance analysis of censored survival data. Biometrics. 1974 Mar;30(1):89–99. [PubMed] [Google Scholar]
- Chiang B. L., Bearer E., Ansari A., Dorshkind K., Gershwin M. E. The BM12 mutation and autoantibodies to dsDNA in NZB.H-2bm12 mice. J Immunol. 1990 Jul 1;145(1):94–101. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cibotti R., Kanellopoulos J. M., Cabaniols J. P., Halle-Panenko O., Kosmatopoulos K., Sercarz E., Kourilsky P. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):416–420. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Creech E., Nakul-Aquaronne D., McDaniel R., Ackler S., Rapoport R. G., Sobel E. S., Eisenberg R. A. Antigen nonspecific effect of major histocompatibility complex haplotype on autoantibody levels in systemic lupus erythematosus-prone lpr mice. J Clin Invest. 1993 Jun;91(6):2761–2768. doi: 10.1172/JCI116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S., Sainis K., Owen F. L., Datta S. K. T-cell-receptor beta- and I-A beta-chain genes of normal SWR mice are linked with the development of lupus nephritis in NZB x SWR crosses. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6850–6853. doi: 10.1073/pnas.84.19.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L. M., Izui S., Dixon F. J. (NZW x BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981 Jul 1;154(1):216–221. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Hudgins C. C., Steinberg R. T., Klinman D. M., Reeves M. J., Steinberg A. D. Studies of consomic mice bearing the Y chromosome of the BXSB mouse. J Immunol. 1985 Jun;134(6):3849–3854. [PubMed] [Google Scholar]
- Izui S., Higaki M., Morrow D., Merino R. The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW x C57BL/6)F1 male mice, but not in C57BL/6 male mice. Eur J Immunol. 1988 Jun;18(6):911–915. doi: 10.1002/eji.1830180612. [DOI] [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Izui S., Masuda K., Yoshida H. Acute SLE in F1 hybrids between SB/Le and NZW mice; prominently enhanced formation of gp70 immune complexes by a Y chromosome-associated factor from SB/Le mice. J Immunol. 1984 Feb;132(2):701–704. [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Clark J. P., Hang L. M., Hara I., Dixon F. J. Retroviral gp70 immune complexes in NZB x NZW F2 mice with murine lupus nephritis. J Exp Med. 1981 Aug 1;154(2):517–528. doi: 10.1084/jem.154.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Abe M., Zhang D., Saikawa T., Fujimori M., Hirose S., Shirai T. Heterozygosity of the major histocompatibility complex controls the autoimmune disease in (NZW x BXSB) F1 mice. Clin Immunol Immunopathol. 1992 Dec;65(3):308–314. doi: 10.1016/0090-1229(92)90162-h. [DOI] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Three genes for lupus nephritis in NZB x NZW mice. J Exp Med. 1978 Jun 1;147(6):1653–1660. doi: 10.1084/jem.147.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Hammerling U., Denaro M., Lund T., Flavell R. A., Rask L., Peterson P. A. Structure of the murine immune response I-A beta locus: sequence of the I-A beta gene and an adjacent beta-chain second domain exon. Cell. 1983 Aug;34(1):179–188. doi: 10.1016/0092-8674(83)90148-4. [DOI] [PubMed] [Google Scholar]
- Luzuy S., Merino J., Engers H., Izui S., Lambert P. H. Autoimmunity after induction of neonatal tolerance to alloantigens: role of B cell chimerism and F1 donor B cell activation. J Immunol. 1986 Jun 15;136(12):4420–4426. [PubMed] [Google Scholar]
- Merino R., Fossati L., Izui S. The lupus-prone BXSB strain: the Yaa gene model of systemic lupus erythematosus. Springer Semin Immunopathol. 1992;14(2):141–157. doi: 10.1007/BF00195291. [DOI] [PubMed] [Google Scholar]
- Merino R., Fossati L., Lacour M., Izui S. Selective autoantibody production by Yaa+ B cells in autoimmune Yaa(+)-Yaa- bone marrow chimeric mice. J Exp Med. 1991 Nov 1;174(5):1023–1029. doi: 10.1084/jem.174.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Fossati L., Lacour M., Lemoine R., Higaki M., Izui S. H-2-linked control of the Yaa gene-induced acceleration of lupus-like autoimmune disease in BXSB mice. Eur J Immunol. 1992 Feb;22(2):295–299. doi: 10.1002/eji.1830220202. [DOI] [PubMed] [Google Scholar]
- Merino R., Iwamoto M., Fossati L., Muniesa P., Araki K., Takahashi S., Huarte J., Yamamura K., Vassalli J. D., Izui S. Prevention of systemic lupus erythematosus in autoimmune BXSB mice by a transgene encoding I-E alpha chain. J Exp Med. 1993 Oct 1;178(4):1189–1197. doi: 10.1084/jem.178.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Shibata T., De Kossodo S., Izui S. Differential effect of the autoimmune Yaa and lpr genes on the acceleration of lupus-like syndrome in MRL/MpJ mice. Eur J Immunol. 1989 Nov;19(11):2131–2137. doi: 10.1002/eji.1830191124. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Hughes J. L., Houghten R., McLachlan A., Jones J. E. Functional identification of agretopic and epitopic residues within an HBcAg T cell determinant. J Immunol. 1989 Nov 15;143(10):3141–3147. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Raney A. K., Houghten R., Thornton G. B., Maruyama T., Hughes J. L., Jones J. E. Autoantibody production in hepatitis B e antigen transgenic mice elicited with a self T-cell peptide and inhibited with nonself peptides. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4348–4352. doi: 10.1073/pnas.88.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. D., Roths J. B. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979 Nov;22(11):1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- Naiki M., Yoshida S. H., Watanabe Y., Izui S., Ansari A. A., Gershwin M. E. The contribution of I-Abm12 to phenotypic and functional alterations among T-cell subsets in NZB mice. J Autoimmun. 1993 Apr;6(2):131–143. doi: 10.1006/jaut.1993.1011. [DOI] [PubMed] [Google Scholar]
- Nygard N. R., McCarthy D. M., Schiffenbauer J., Schwartz B. D. Mixed haplotypes and autoimmunity. Immunol Today. 1993 Feb;14(2):53–56. doi: 10.1016/0167-5699(93)90058-s. [DOI] [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen J. L., Klippel J. H., Johnson A. H., Steinberg A. D., Decker J. L., Mann D. L. B-lymphocyte alloantigens associated with systemic lupus erythematosus. N Engl J Med. 1978 Sep 7;299(10):515–518. doi: 10.1056/NEJM197809072991004. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Murphy E. D., Eicher E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984 Jan 1;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]


