Abstract
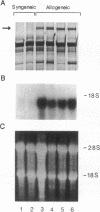
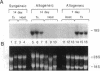
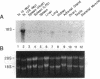
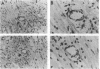
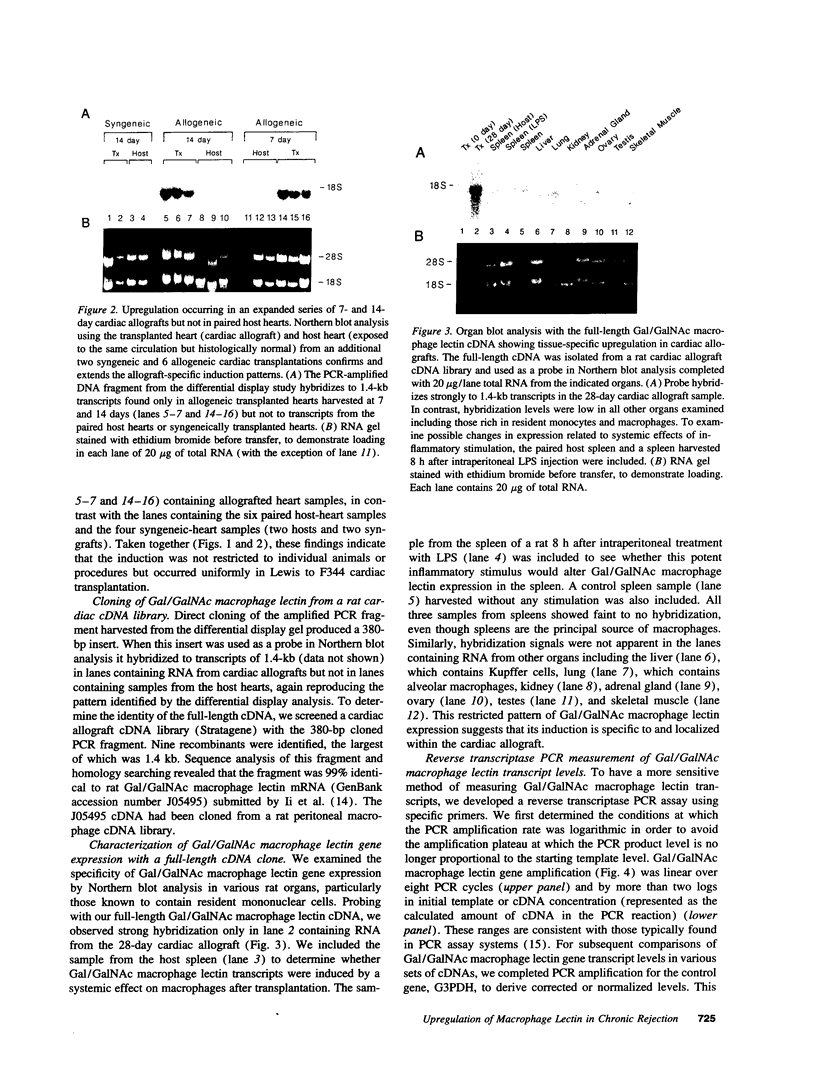
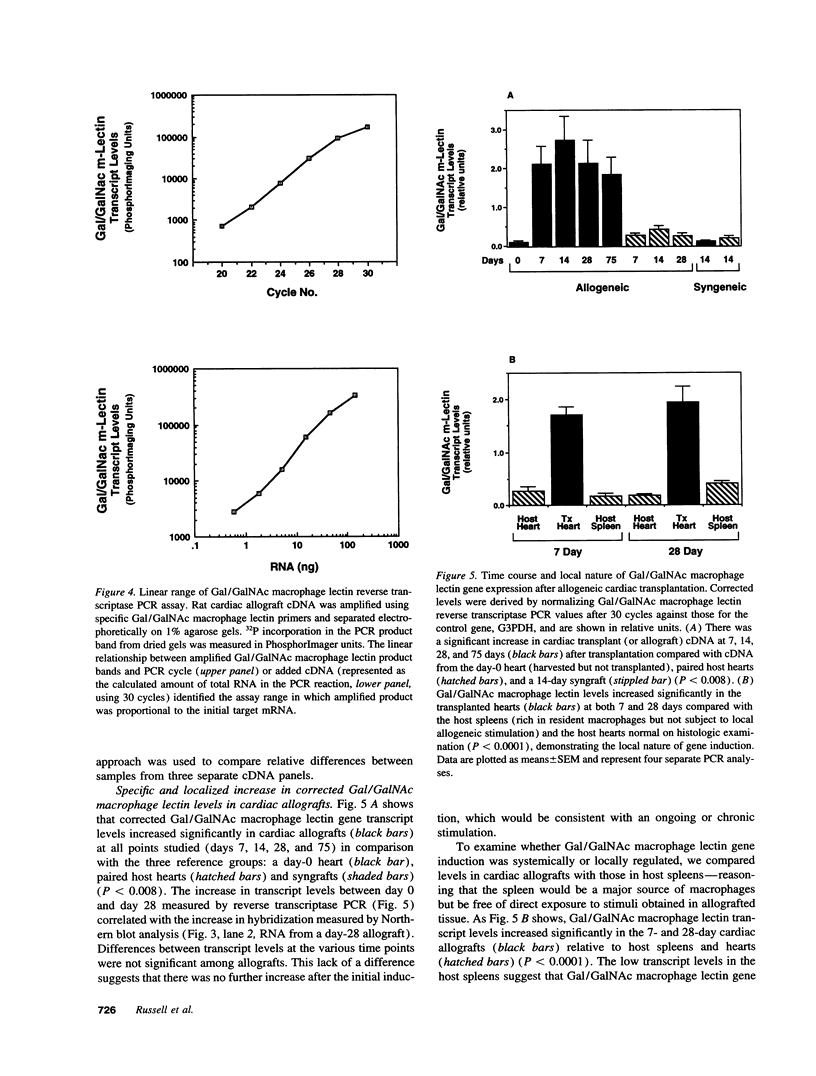
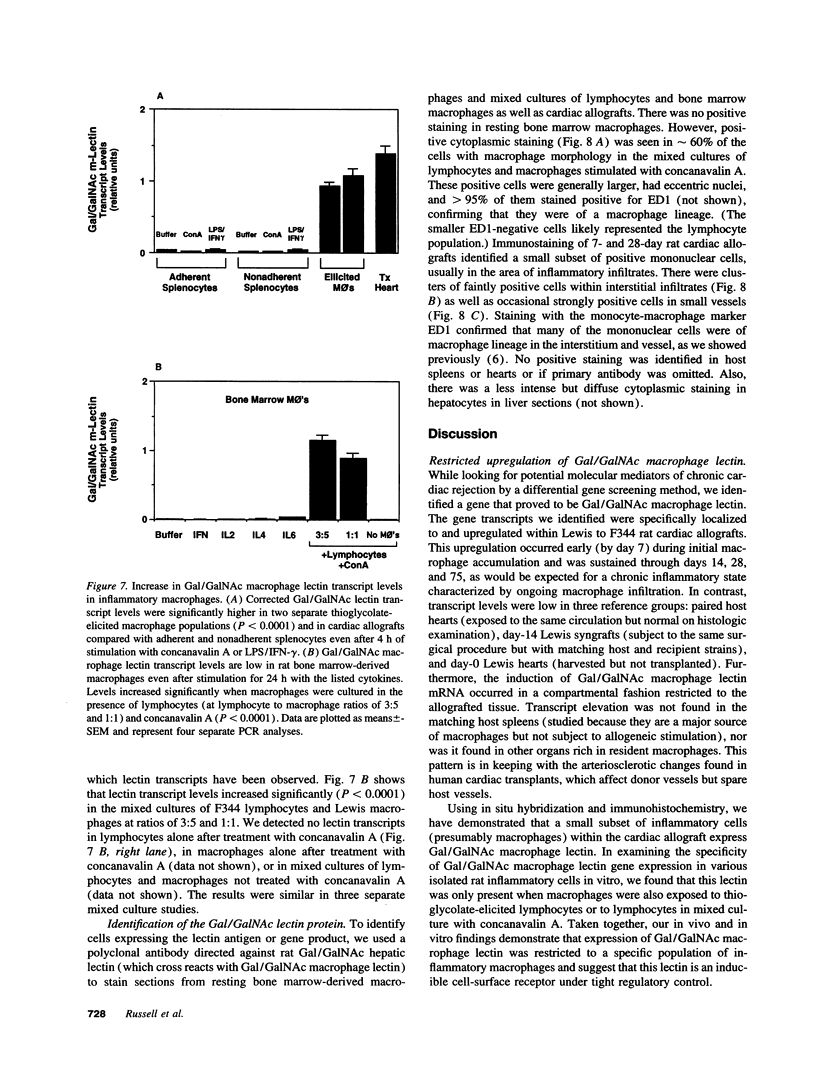
Using differential mRNA display to uncover potential mediators associated with chronic rejection, we identified a cDNA fragment induced in Lewis to F344 rat cardiac allografts with arteriosclerosis but not Lewis syngrafts. The full-length cDNA (1.4 kb) isolated from a rat cardiac allograft cDNA library was 99% identical to galactose/N-acetylgalactosamine (Gal/GalNAc) macrophage lectin, a cell-surface receptor. This cDNA hybridized in Northern analysis with total RNA from eight cardiac allografts but not with host hearts, syngrafts, or other organs. There was a significant allograft-specific increase in transcript levels measured by reverse transcriptase PCR at days 7, 14, 28, and 75 in comparison with paired F344 host hearts (subject to same circulation but histologically normal), day-0 hearts, and syngrafts (P < 0.008, n = 4 at each time). Transcript levels in cardiac allografts were higher than those in paired host spleens (a major source of inflammatory cells) (P < 0.0001), indicating the localized nature of Gal/GalNAc lectin induction. By in situ hybridization and immunostaining, Gal/GalNAc lectin expression localized to a subset of inflammatory cells in cardiac allografts. These findings link Gal/GalNAc macrophage lectin to the chronic rejection process, as a possible mediator of macrophage infiltration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Tilney N. L., Collins J. J., Jr, Karnovsky M. J. Experimental graft arteriosclerosis. I. The Lewis-to-F-344 allograft model. Transplantation. 1992 May;53(5):1115–1119. [PubMed] [Google Scholar]
- Adams D. H., Wyner L. R., Karnovsky M. J. Experimental graft arteriosclerosis. II. Immunocytochemical analysis of lesion development. Transplantation. 1993 Oct;56(4):794–799. doi: 10.1097/00007890-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Arceci R. J., King A. A., Simon M. C., Orkin S. H., Wilson D. B. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993 Apr;13(4):2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Wiltschke C., Holzinger C., Fellinger A., Scheiner O., Gessl A., Förster O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol. 1987 Jan;41(1):83–91. doi: 10.1002/jlb.41.1.83. [DOI] [PubMed] [Google Scholar]
- Cramer D. V., Wu G. D., Chapman F. A., Cajulis E., Wang H. K., Makowka L. Lymphocytic subsets and histopathologic changes associated with the development of heart transplant arteriosclerosis. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 1):458–466. [PubMed] [Google Scholar]
- Ii M., Kawasaki T., Yamashina I. Structural similarity between the macrophage lectin specific for galactose/N-acetylgalactosamine and the hepatic asialoglycoprotein binding protein. Biochem Biophys Res Commun. 1988 Sep 15;155(2):720–725. doi: 10.1016/s0006-291x(88)80554-0. [DOI] [PubMed] [Google Scholar]
- Ii M., Kurata H., Itoh N., Yamashina I., Kawasaki T. Molecular cloning and sequence analysis of cDNA encoding the macrophage lectin specific for galactose and N-acetylgalactosamine. J Biol Chem. 1990 Jul 5;265(19):11295–11298. [PubMed] [Google Scholar]
- Kawasaki T., Ii M., Kozutsumi Y., Yamashina I. Isolation and characterization of a receptor lectin specific for galactose/N-acetylgalactosamine from macrophages. Carbohydr Res. 1986 Aug 15;151:197–206. doi: 10.1016/s0008-6215(00)90340-9. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Oda S., Sato M., Toyoshima S., Osawa T. Binding of activated macrophages to tumor cells through a macrophage lectin and its role in macrophage tumoricidal activity. J Biochem. 1989 Jun;105(6):1040–1043. doi: 10.1093/oxfordjournals.jbchem.a122763. [DOI] [PubMed] [Google Scholar]
- Oda S., Sato M., Toyoshima S., Osawa T. Purification and characterization of a lectin-like molecule specific for galactose/N-acetyl-galactosamine from tumoricidal macrophages. J Biochem. 1988 Oct;104(4):600–605. doi: 10.1093/oxfordjournals.jbchem.a122518. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Ii M., Itoh N., Kawasaki T. Expression of a functional asialoglycoprotein receptor through transfection of a cloned cDNA that encodes a macrophage lectin. J Biol Chem. 1992 May 5;267(13):9229–9235. [PubMed] [Google Scholar]
- Russell M. E., Adams D. H., Wyner L. R., Yamashita Y., Halnon N. J., Karnovsky M. J. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Sharples L. D., Caine N., Mullins P., Scott J. P., Solis E., English T. A., Large S. R., Schofield P. M., Wallwork J. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991 Aug;52(2):244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Steinbeck M. J., Khan A. U., Karnovsky M. J. Extracellular production of singlet oxygen by stimulated macrophages quantified using 9,10-diphenylanthracene and perylene in a polystyrene film. J Biol Chem. 1993 Jul 25;268(21):15649–15654. [PubMed] [Google Scholar]