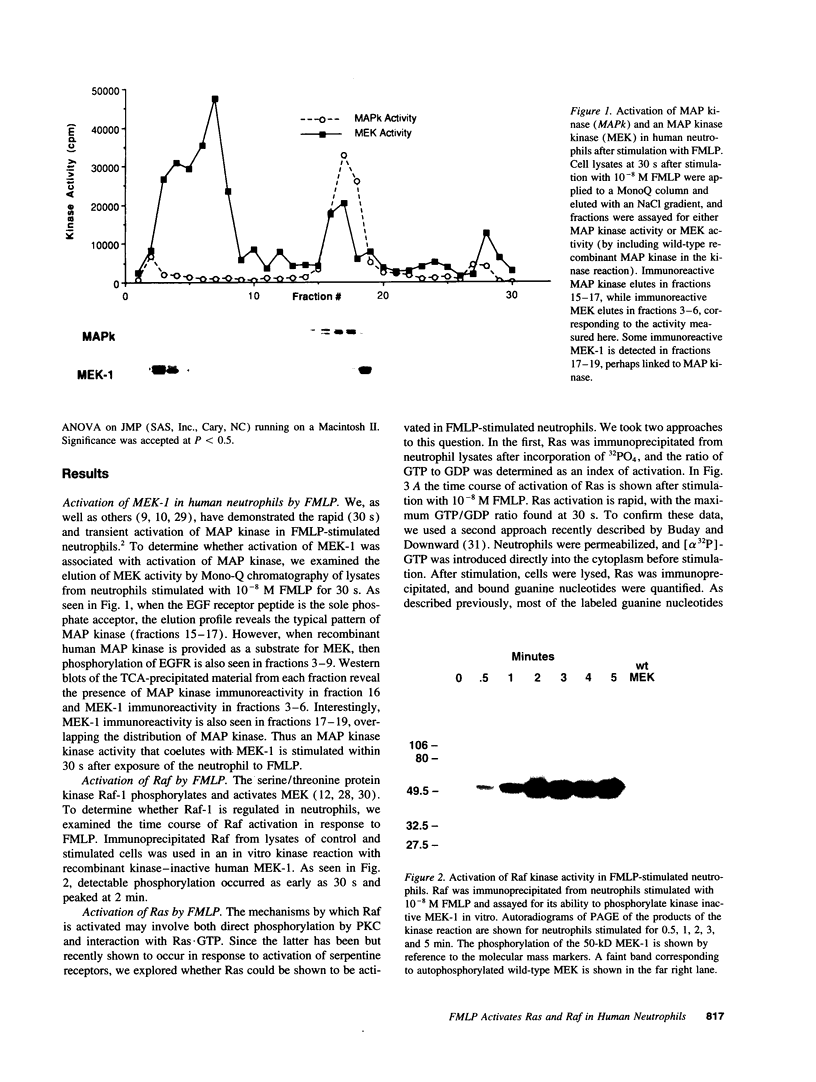
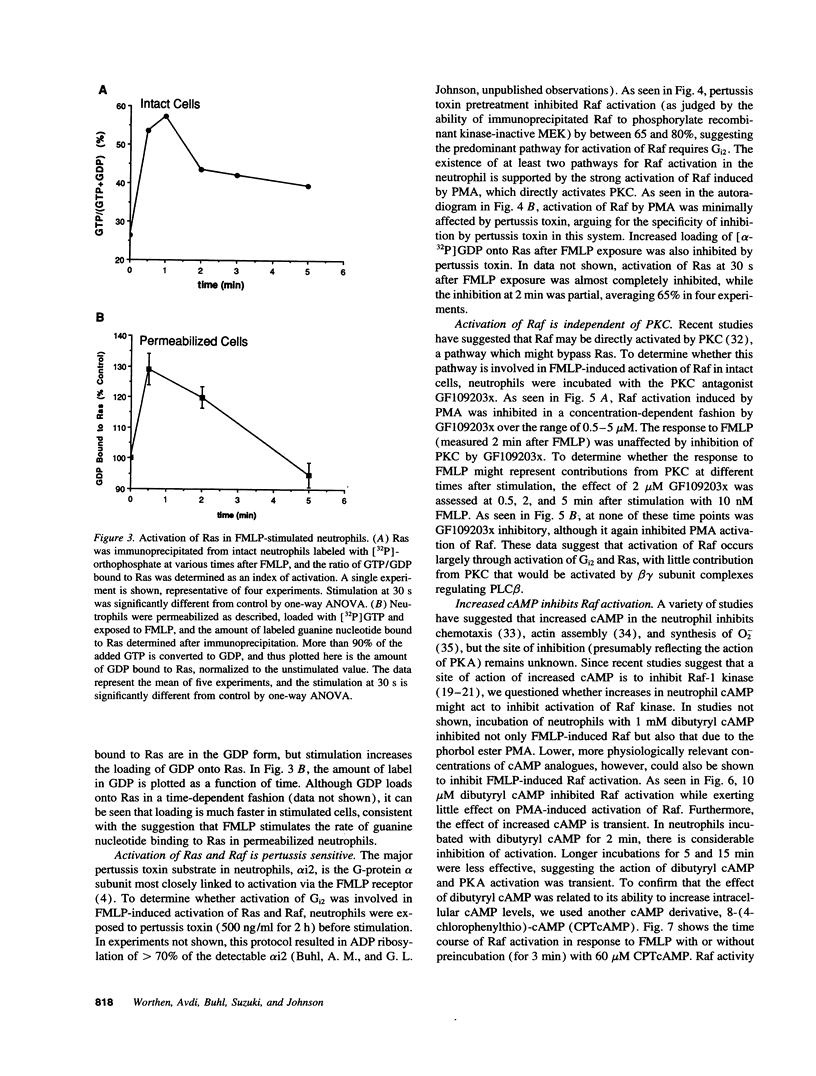
Abstract
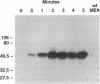
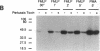
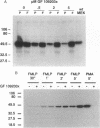
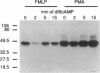
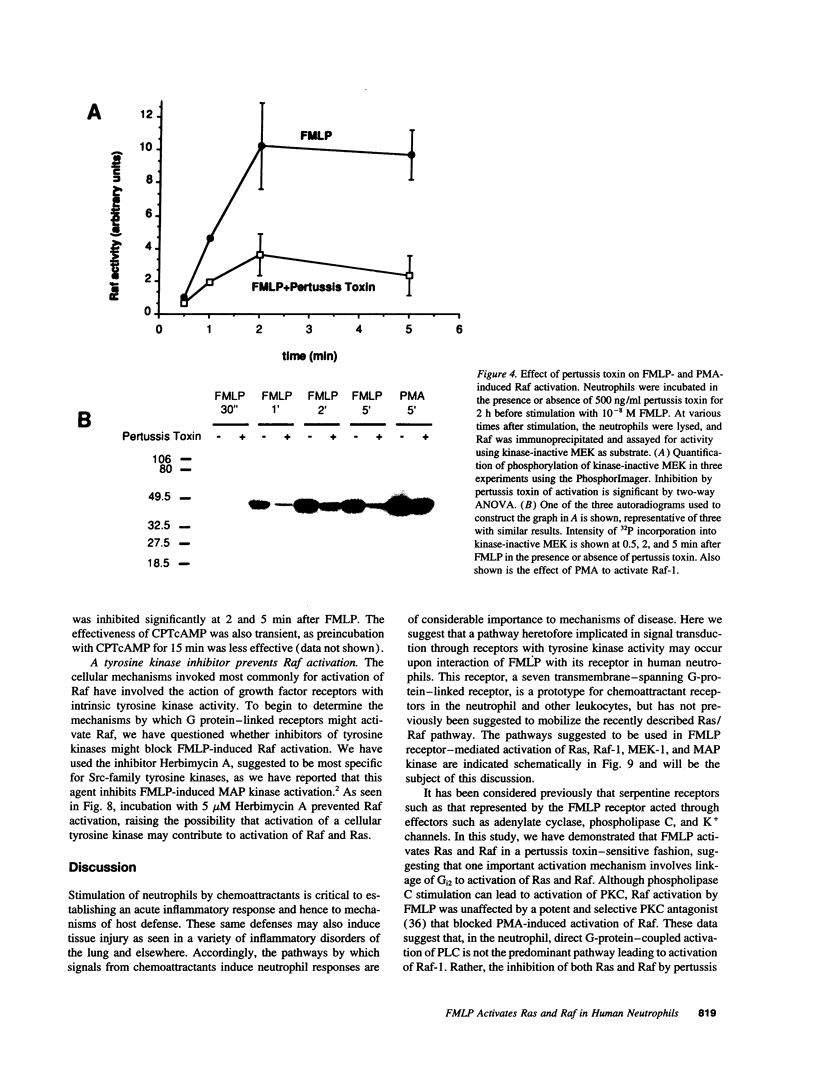
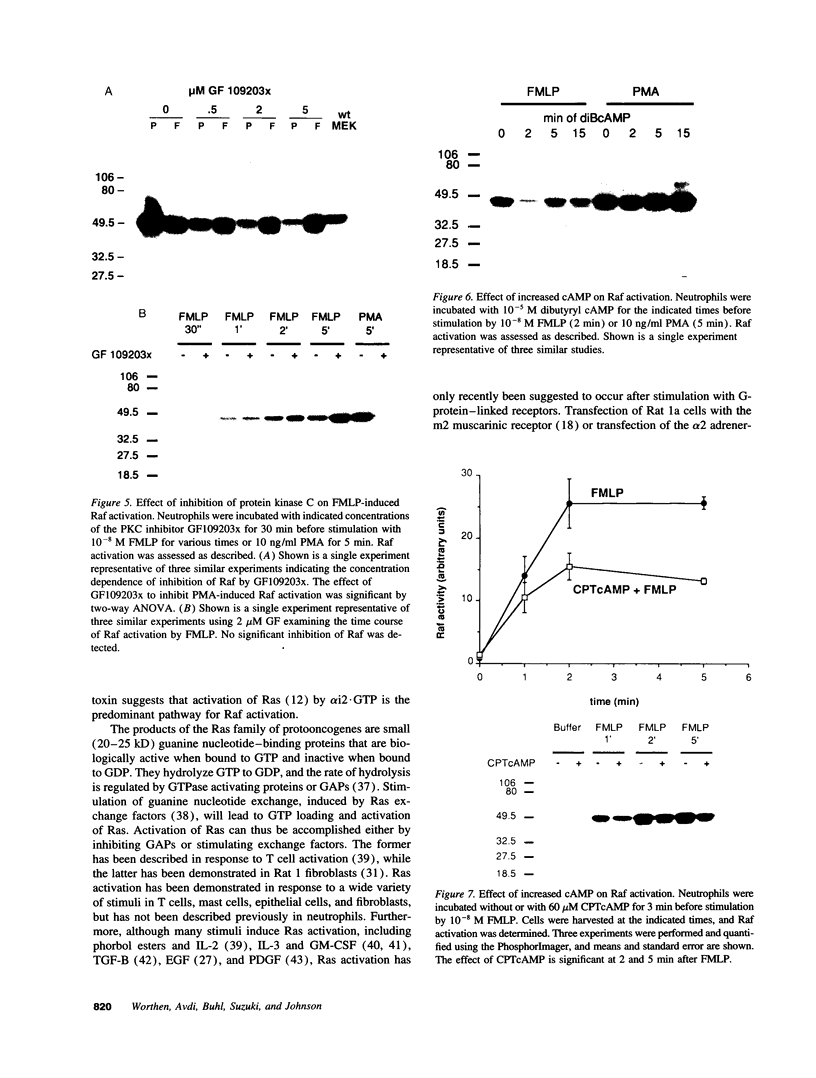
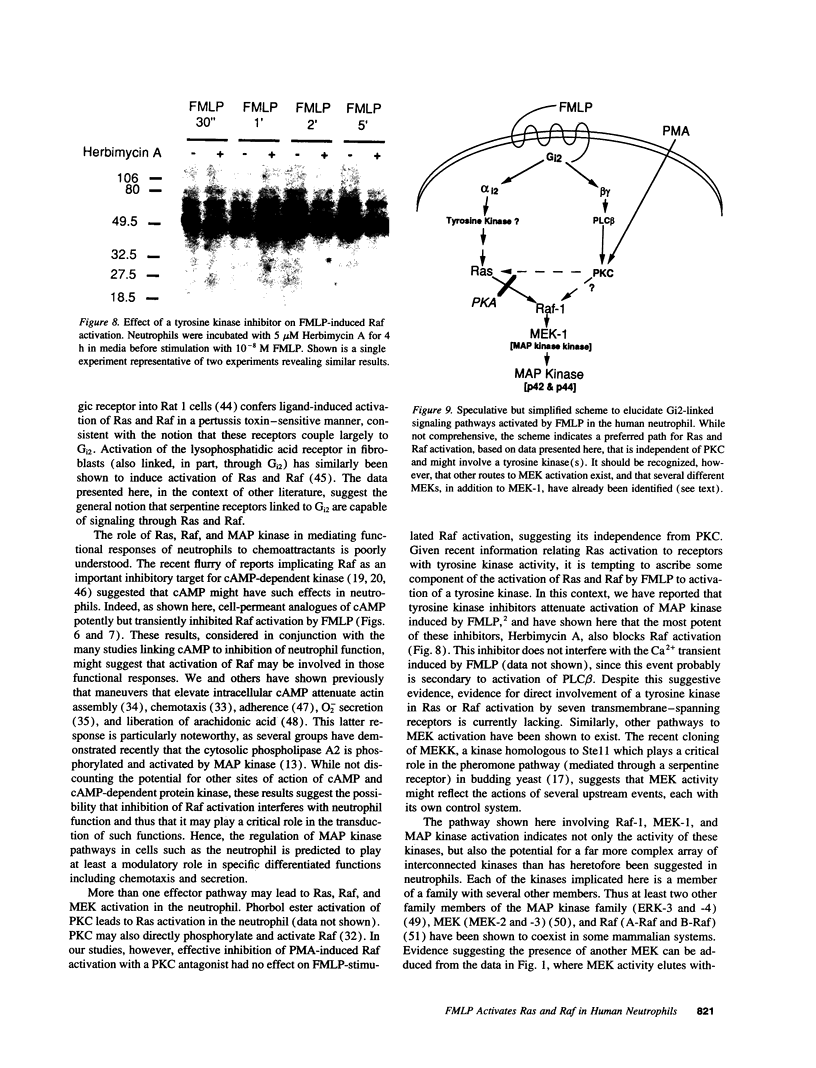
Chemoattractants bind to seven transmembrane-spanning, G-protein-linked receptors on polymorphonuclear leukocytes (neutrophils) and induce a variety of functional responses, including activation of microtubule-associated protein (MAP) kinase. Although the pathways by which MAP kinases are activated in neutrophils are unknown, we hypothesized that activation of the Ras/Raf pathway leading to activation of MAP/ERK kinase (MEK) would be induced by the chemoattractant f-met-leu-phe. Human neutrophils exposed to 10 nM FMLP for 30 s exhibited an MAP kinase kinase activity coeluting with MEK-1. Immunoprecipitation of Raf-1 kinase after stimulation with FMLP revealed an activity that phosphorylated MEK, was detectable at 30 s, and peaked at 2-3 min. Immunoprecipitation of Ras from both intact neutrophils labeled with [32P]orthophosphate and electropermeabilized neutrophils incubated with [32P]GTP was used to determine that FMLP treatment was associated with activation of Ras. Activation of both Ras and Raf was inhibited by treatment of neutrophils with pertussis toxin, indicating predominant linkage to the Gi2 protein. Although phorbol esters activated Raf, activation induced by FMLP appeared independent of protein kinase C, further suggesting that Gi2 was linked to Ras and Raf independent of phospholipase C and protein kinase C. Dibutyryl cAMP, which inhibits many neutrophil functional responses, blocked the activation of Raf by FMLP, suggesting that interruption of the Raf/MAP kinase pathway influences neutrophil responses to chemoattractants. These data suggest that Gi2-mediated receptor regulation of the Ras/Raf/MAP kinase pathway is a primary response to chemoattractants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alblas J., van Corven E. J., Hordijk P. L., Milligan G., Moolenaar W. H. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by alpha 2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993 Oct 25;268(30):22235–22238. [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates the exchange rate of guanine nucleotides on p21ras in fibroblasts. Mol Cell Biol. 1993 Mar;13(3):1903–1910. doi: 10.1128/mcb.13.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Pronk G. J., van Weeren P. C., Chardin P., Bos J. L. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 1993 Nov;12(11):4211–4220. doi: 10.1002/j.1460-2075.1993.tb06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Chopra J., Webster R. O. PGE1 inhibits neutrophil adherence and neutrophil-mediated injury to cultured endothelial cells. Am Rev Respir Dis. 1988 Oct;138(4):915–920. doi: 10.1164/ajrccm/138.4.915. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. The receptors for ATP and fMetLeuPhe are independently coupled to phospholipases C and A2 via G-protein(s). Relationship between phospholipase C and A2 activation and exocytosis in HL60 cells and human neutrophils. Biochem J. 1989 Nov 1;263(3):715–723. doi: 10.1042/bj2630715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Elson E. L., Schwab B., 3rd, Erzurum S. C., Young S. K., Worthen G. S. Biophysical properties and microfilament assembly in neutrophils: modulation by cyclic AMP. J Cell Biol. 1991 Sep;114(6):1179–1190. doi: 10.1083/jcb.114.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Riehl R., Wu L., Weinberg R. A. Identification of a nucleotide exchange-promoting activity for p21ras. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5998–6002. doi: 10.1073/pnas.87.15.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. The ras superfamily of small GTP-binding proteins. Trends Biochem Sci. 1990 Dec;15(12):469–472. doi: 10.1016/0968-0004(90)90300-z. [DOI] [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Marasco W. A., Elgas L. J., Ward P. A. Stimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion production. Am J Pathol. 1984 Apr;115(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Fonteh A. N., Winkler J. D., Torphy T. J., Heravi J., Undem B. J., Chilton F. H. Influence of isoproterenol and phosphodiesterase inhibitors on platelet-activating factor biosynthesis in the human neutrophil. J Immunol. 1993 Jul 1;151(1):339–350. [PubMed] [Google Scholar]
- Gardner A. M., Vaillancourt R. R., Johnson G. L. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993 Aug 25;268(24):17896–17901. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992 Sep 5;267(25):18122–18125. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Receptor-mediated activation of electropermeabilized neutrophils. Evidence for a Ca2+- and protein kinase C-independent signaling pathway. J Biol Chem. 1988 Feb 5;263(4):1779–1783. [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L., Heasley L. E. MAP kinase is constitutively activated in gip2 and src transformed rat 1a fibroblasts. J Biol Chem. 1992 Apr 25;267(12):7987–7990. [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Howe L. R., Marshall C. J. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993 Oct 5;268(28):20717–20720. [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993 Jan 25;268(3):1960–1964. [PubMed] [Google Scholar]
- Pelech S. L. Networking with protein kinases. Curr Biol. 1993 Aug 1;3(8):513–515. doi: 10.1016/0960-9822(93)90043-n. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Heidecker G., Huleihel M., Cleveland J. L., Choi W. C., Pawson T., Ihle J. N., Anderson W. B. raf family serine/threonine protein kinases in mitogen signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):173–184. doi: 10.1101/sqb.1988.053.01.023. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Sandborg R. R., Smolen J. E. Early biochemical events in leukocyte activation. Lab Invest. 1988 Sep;59(3):300–320. [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Nakamura S., Kaziro Y. Platelet-derived growth factor stimulates formation of active p21ras.GTP complex in Swiss mouse 3T3 cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5993–5997. doi: 10.1073/pnas.87.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Seuwen K., Kahan C., Hartmann T., Pouyssegur J. Strong and persistent activation of inositol lipid breakdown induces early mitogenic events but not Go to S phase progression in hamster fibroblasts. Comparison of thrombin and carbachol action in cells expressing M1 muscarinic acetylcholine receptors. J Biol Chem. 1990 Dec 25;265(36):22292–22299. [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. M., Pyun H. Y., Navarro J. Molecular cloning of the fMet-Leu-Phe receptor from neutrophils. J Biol Chem. 1990 Nov 25;265(33):20061–20064. [PubMed] [Google Scholar]
- Thompson H. L., Shiroo M., Saklatvala J. The chemotactic factor N-formylmethionyl-leucyl-phenylalanine activates microtubule-associated protein 2 (MAP) kinase and a MAP kinase kinase in polymorphonuclear leucocytes. Biochem J. 1993 Mar 1;290(Pt 2):483–488. doi: 10.1042/bj2900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Hall F. L., O'Neill K. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. J Immunol. 1993 Feb 15;150(4):1563–1577. [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Winitz S., Russell M., Qian N. X., Gardner A., Dwyer L., Johnson G. L. Involvement of Ras and Raf in the Gi-coupled acetylcholine muscarinic m2 receptor activation of mitogen-activated protein (MAP) kinase kinase and MAP kinase. J Biol Chem. 1993 Sep 15;268(26):19196–19199. [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Yatani A., Mattera R., Codina J., Graf R., Okabe K., Padrell E., Iyengar R., Brown A. M., Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Gi alpha-subunits. Nature. 1988 Dec 15;336(6200):680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993 Nov 15;268(32):23933–23939. [PubMed] [Google Scholar]