Abstract
Weight gain during pregnancy may contribute to increased urinary incontinence (UI) during and after pregnancy, but scientific support is lacking. The effect of weight loss on UI postpartum is unclear. From 1999 to 2006, investigators in the Norwegian Mother and Child Cohort Study recruited pregnant women during pregnancy. This study was based on 12,679 primiparous women who were continent before pregnancy. Data were obtained from questionnaires answered at weeks 15 and 30 of pregnancy and 6 months postpartum. Weight gain greater than the 50th percentile during weeks 0–15 of pregnancy was weakly associated with higher incidence of UI at week 30 compared with weight gain less than or equal to the 50th percentile. Weight gain greater than the 50th percentile during pregnancy was not associated with increased prevalence of UI 6 months postpartum. For each kilogram of weight loss from delivery to 6 months postpartum among women who were incontinent during pregnancy, the relative risk for UI decreased 2.1% (relative risk = 0.98, 95% confidence interval: 0.97, 0.99). Weight gain during pregnancy does not seem to be a risk factor for increased incidence or prevalence of UI during pregnancy or postpartum. However, weight loss postpartum may be important for avoiding incontinence and regaining continence 6 months postpartum.
Keywords: cohort studies, parity, postpartum period, pregnancy, urinary incontinence, weight gain, weight loss
Urinary incontinence (UI) is a common condition among women (1–4). The etiology is multifactorial, but pregnancy and delivery seem to be major risk factors, especially among young and middle-aged women (5, 6). A high body mass index (BMI) is an established risk factor for UI (7). Weight gain also shows strong associations with UI (8, 9). High weight gain during pregnancy is believed to contribute significantly to the increase of UI during and after pregnancy, but epidemiologic studies assessing this relation have been inconclusive (10–19). All identified studies have considerable limitations, and none was designed to assess the association between UI and weight gain. Weight loss is documented to reduce UI in obese women (20), but no studies have specifically investigated how UI is affected by weight loss postpartum.
Given the limitations of the previous research, we decided to perform an analysis based on the Norwegian Mother and Child Cohort Study (MoBa), a large population-based cohort study of pregnant women with several years of follow-up investigating health issues among mothers and children (21). The population for the present substudy consisted of primigravid women who were continent before pregnancy. Our objectives were to investigate how the incidence of UI during pregnancy was affected by weight gain during weeks 0–15 and 15–30 of pregnancy. We also investigated how incidence and prevalence of UI 6 months postpartum was affected by weight gain during weeks 0–15, 15–30, 30–delivery, and 0–delivery; by weight gain from week 0 of pregnancy to 6 months postpartum; and by weight loss from delivery to 6 months postpartum.
MATERIALS AND METHODS
The Norwegian Mother and Child Cohort Study
There are approximately 55,000 births annually in Norway. Each year since 1999, MoBa investigators have invited approximately 30,000 pregnant women, recruited from 39 of 50 Norwegian maternity units with more than 100 births annually, to enroll. The pregnant women were invited by mail 3 weeks before undergoing their routine pregnancy ultrasound examination at gestational week 18 (21). By 2006, 43.2% of the invited primiparous women had agreed to participate by informed written consent (22). The enrollment of participants has been completed, since MoBa has met the target number of 100,000 pregnant women. Follow-up is still ongoing.
To explore causative mechanisms for disease among mothers and children, MoBa investigators obtained data by means of postal questionnaires at 6 time points, from week 15 in pregnancy to 3 years after birth. We used a data set from 1999–2006 based on questionnaire 1, distributed during week 15 of pregnancy; questionnaire 3, distributed during week 30; and questionnaire 4, distributed 6 months postpartum. The questionnaires are available on the Journal's Web site (http://aje.oxfordjournals.org/). Women were asked to participate only once. However, given participation, responses in the follow-up studies were strongly emphasized. Questionnaires 3 and 4 were completed by 92% and 87%, respectively, of the women who were enrolled in MoBa (21). Data from MoBa, which is conducted by the Norwegian Institute of Public Health (21), were linked with data from the Medical Birth Registry of Norway, where data on all deliveries taking place in Norway have been registered since 1967.
The Norwegian Data Inspectorate approved MoBa in 1996, renewed the approval in 2003, and approved the linkage of the MoBa data set with that of the Medical Birth Registry of Norway. The Regional Ethics Committee for Medical Research in Health Region II endorsed the project.
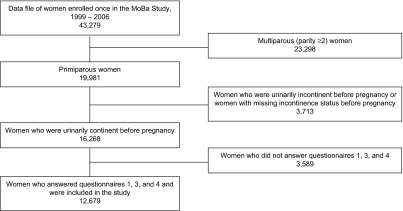
We designed a substudy to explore the specific association between UI and weight change during pregnancy and postpartum. We included women who were primiparous, continent before pregnancy, and having singletons. Women who had not completed questionnaire 1, questionnaire 3, and/or questionnaire 4 were excluded. Thus, our study population consisted of 12,679 women (Figure 1). The 3,589 excluded women did not differ significantly from the 12,679 included women with regard to outcome and exposure variables, so our sample was not biased in this respect. Descriptive data from the data set have been published previously (23).
Figure 1.
Selection of participants for a study of weight change during and after pregnancy and urinary incontinence, Norwegian Mother and Child Cohort Study (MoBa), 1999–2006.
Urinary incontinence
Incidence of UI in week 30 of pregnancy and incidence and prevalence of UI 6 months postpartum were the main outcomes in this study. We used a questionnaire based on the definitions of the International Continence Society (24). In questionnaire 1, women reported UI before pregnancy. In questionnaires 3 and 4, the women reported UI at week 30 of pregnancy and 6 months postpartum, respectively. Women were asked about UI that occurred when coughing/laughing/sneezing, when running or jumping, or as leakage accompanied by a strong urgency to void. Women who confirmed loss of urine were defined as having “any UI” according to the standardized terminology of urinary tract symptoms (24).
BMI, weight, and weight change
Height was reported on questionnaire 1. Weight was reported at weeks 0 and 15 of pregnancy (questionnaire 1), at week 30 of pregnancy (questionnaire 3), and at delivery and 6 months postpartum (questionnaire 4). Weight was reported on questionnaire 1 without decimals; the remainder of weight reports included 1 decimal digit.
The exposures evaluated in this study were weight gain during weeks 0–15, 15–30, 30–delivery, and 0–delivery; weight gain from week 0 of pregnancy to 6 months postpartum; and weight loss from delivery to 6 months postpartum. We investigated the associations between UI at week 30 of pregnancy and weight gain during weeks 0–15 and 15–30. We investigated the association between UI 6 months postpartum and weight gain for the following time periods: weeks 0–15, 15–30, 30–delivery, and 0–delivery and week 0–6 months postpartum. In addition, we investigated the association between weight loss from delivery to 6 months postpartum and persistent UI 6 months postpartum among women who were incontinent at week 30, and incident UI 6 months postpartum among women who were continent at week 30.
BMI was calculated as weight in kilograms divided by the square of height in meters. For stratified analyses using prepregnancy BMI, we excluded women who did not report their prepregnancy weight or height on questionnaire 1 (n = 447). Prepregnancy BMI was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
The 50th and 90th percentiles for weight gain during each time period were used to categorize weight gain (1%–50%, 51%–90%, and >90%). Expectedly, a large number of women had the exact weight corresponding to the median weight change (50th percentile); hence, the weight groups do not correspond exactly to the intended percentiles. Women with weight gain representing the 50th or 90th percentile were included in the 1–50th or 51–90th percentile group, respectively. The term “high weight gain” refers to weight gain beyond the 50th percentile.
For the 12,679 women who intended to report weight at 5 time points, 1,578 women had a total of 2,350 missing data points on weight (3.7%). All reported weights were used in the analyses. Outliers for height were excluded (<100 cm; 34 data points), leaving values of 140–190 cm. Outliers for weight were excluded (<25 kg and >400 kg; 34 data points), leaving values of 40–180 kg. Outliers for weight change during the set time periods were excluded (>50 kg; 6 data points). To isolate the effect of weight gain, we excluded data points representing weight loss during the set time periods of pregnancy (1,860 data points). To isolate the effect of weight loss, we excluded data points representing weight gain from delivery to 6 months postpartum (30 data points). All weight changes, including weight loss, were included in the analyses regarding weight change from week 0 of pregnancy to 6 months postpartum.
Percentage of weight change, BMI change, weight gain according to the US Institute of Medicine recommendations (25), and absolute weight change were explored. These methods led to the same patterns of results. We present both absolute weight change in kilograms at the 50th and 90th percentiles and weight change as a continuous variable, since we found these methods appropriate for our study aim and easy to interpret.
Effect modification and confounding
Effect modification by BMI of the association between weight change and UI was tested using the Breslow-Day test for homogeneity between odds ratios after stratified analyses (26) and by logistic regression analyses. None of the analyses suggested significant effect modification. Results stratified for BMI are presented because of their potential clinical relevance.
Confounding was evaluated by means of multivariable logistic regression and cross-tab analyses. The following potential confounders were explored for each appropriate time period: age, mode of delivery (cesarean section, vaginal delivery), UI during pregnancy, baby's head circumference, baby's birth weight, fetal presentation at delivery (normal occipital, breech, transverse, abnormal fetal head presentation, or other), perineal tear grade 3–4, breastfeeding (number of months), and physical exercise (sessions/week).
Statistical analyses
Cumulative incidence of UI during pregnancy was estimated as any UI at week 30 of pregnancy. Cumulative incidence of UI postpartum was estimated as any UI 6 months postpartum among women who were continent at week 30 of pregnancy. We treated independent variables as categorical, whereas weight gain and weight loss were also treated as continuous variables in logistic regression analyses. We evaluated the assumption of a linear trend between weight change and UI through model comparisons using chi-squared tests. In the categorical model, we used quartiles for weight change. The assumption of linearity could not be rejected (P = 0.65 for weight gain and P = 0.06 for weight loss). Adjustment for confounding was done by multivariable logistic regression for the different time periods (27). SPSS 15.0 for Windows (SPSS, Inc., Chicago, Illinois) was used for the general statistical analyses. We used Intercooled STATA 9.0 (Stata Corporation, College Station, Texas) for log binomial regression analyses in order to present risk parameters as relative risks. (The relative risk is the preferred risk parameter in studies with a high prevalence in the unexposed group.) P values less than 5% were considered statistically significant. Data are presented as mean values, relative risks, and corresponding 95% confidence intervals, stratified by prepregnancy BMI.
RESULTS
Baseline data are presented in Table 1 by BMI group. Overweight women had significantly more cesarean sections than normal-weight women. Underweight women were significantly younger and had fewer cesarean sections than normal-weight women. There was a significantly higher risk of UI among overweight women compared with underweight women both at week 30 of pregnancy (relative risk (RR) = 1.2, 95% confidence interval (CI): 1.0, 1.3) and 6 months postpartum (RR = 1.4, 95% CI: 1.2, 1.6).
Table 1.
Baseline Pregnancy-related Data on 12,679 Participants From the Norwegian Mother and Child Cohort Study, 1999–2006
| All Women | BMI Groupa | |||||||||||
| Underweight | Normal Weight | Overweight | ||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Total no. and % of women | 12,679 | 100 | 427 | 3 | 8,342 | 66 | 3,463 | 27 | ||||
| Age, years | 27.6 (4.3) | 25.9 (4.8) | 27.6 (4.2) | 27.7 (4.4) | ||||||||
| Cesarean section (no. and % of all births) | 1,815 | 14 | 35 | 8 | 1,010 | 12 | 700 | 20 | ||||
| Birth weight, kg | 3.53 (0.55) | 3.34 (0.53) | 3.50 (0.52) | 3.62 (0.58) | ||||||||
| Prepregnancy weight, kg | 67.1 (12.2) | 50.3 (3.9) | 62.0 (6.4) | 81.3 (11.5) | ||||||||
| Prepregnancy BMIb | 23.7 (4.1) | 17.8 (0.7) | 21.9 (1.7) | 28.8 (3.6) | ||||||||
| Weight change during and after pregnancy, kg | ||||||||||||
| Week 0–15 | 3.3 (2.5) | 3.9 (2.7) | 3.3 (2.4) | 3.3 (2.6) | ||||||||
| Week 15–30 | 7.0 (3.1) | 6.8 (2.8) | 7.1 (3.1) | 6.7 (3.3) | ||||||||
| Week 30–delivery | 6.3 (3.5) | 5.9 (3.2) | 6.2 (3.3) | 6.7 (3.7) | ||||||||
| Week 0–delivery | 15.8 (5.9) | 16.2 (5.5) | 15.9 (5.5) | 15.4 (6.7) | ||||||||
| Week 0–6 months PP | 1.2 (5.0) | 2.6 (4.1) | 1.3 (4.4) | 0.7 (6.4) | ||||||||
| Delivery–6 months PP | −14.5 (5.2) | −13.5 (4.5) | −14.6 (4.8) | −14.4 (6.0) | ||||||||
| UI | ||||||||||||
| Incident UI at week 30 | 5,102 | 40 | 162 | 38 | 3,225 | 39 | 1,522 | 44 | ||||
| Incident UI 6 months PPb | 1,562 | 21 | 50 | 19 | 1,007 | 20 | 451 | 23 | ||||
| Prevalence of UI 6 months PP | 3,991 | 32 | 110 | 26 | 2,495 | 30 | 1,240 | 36 | ||||
Abbreviations: BMI, body mass index; PP, postpartum; SD, standard deviation; UI, urinary incontinence.
Data were stratified by prepregnancy BMI (weight (kg)/height (m)2), which was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
Cumulative incidence in women who were continent before pregnancy and in week 30 of pregnancy.
UI at week 30 of pregnancy
High weight gain (>4 kg) during weeks 0–15 was associated with an increased incidence of UI at week 30 of pregnancy among all women and in each BMI group (Table 2). Normal-weight and overweight women whose weight gain was greater than the 90th percentile (≥7 kg and ≥8 kg, respectively) had a significantly increased risk of UI (RR = 1.2). Underweight women had a nonsignificant relative risk of 1.6 for UI when weight gain exceeded the 90th percentile (≥9 kg).
Table 2.
Relative Risk of Urinary Incontinence Among Primiparous Women in Week 30 of Pregnancy, by Weight Gain During Weeks 0–15 and Weeks 15–30, Norwegian Mother and Child Cohort Study, 1999–2006
| Body Mass Indexa Group and Percentile | Weight Gain, kg | Total No. | Urinary Incontinence | Adjusted Relative Riskb | 95% Confidence Interval | |
| No. | % | |||||
| Weeks 0–15 | ||||||
| All women | ||||||
| 1–50 | 0–3 | 6,407 | 2,445 | 38 | 1 | Reference |
| 51–90 | 4–6 | 3,167 | 1,343 | 42 | 1.1* | 1.0, 1.2 |
| >90 | ≥7 | 1,060 | 480 | 45 | 1.2* | 1.1, 1.3 |
| Underweight | ||||||
| 1–50 | 0–3 | 219 | 75 | 34 | 1 | Reference |
| 51–90 | 4–8 | 158 | 62 | 39 | 1.2 | 0.9, 1.5 |
| >90 | ≥9 | 28 | 15 | 54 | 1.6* | 1.1, 2.3 |
| Normal weight | ||||||
| 1–50 | 0–3 | 4,449 | 1,638 | 37 | 1 | Reference |
| 51–90 | 4–6 | 2,287 | 951 | 42 | 1.1* | 1.1, 1.2 |
| >90 | ≥7 | 701 | 303 | 43 | 1.2* | 1.1, 1.3 |
| Overweight | ||||||
| 1–50 | 0–3 | 1,670 | 719 | 43 | 1 | Reference |
| 51–90 | 4–7 | 856 | 389 | 45 | 1.1 | 1.0, 1.2 |
| >90 | ≥8 | 176 | 92 | 52 | 1.2* | 1.0, 1.3 |
| Weeks 15–30 | ||||||
| All women | ||||||
| 1–50 | 0–7.0 | 6,537 | 2,634 | 40 | 1 | Reference |
| 51–90 | 7.1–10.9 | 3,811 | 1,550 | 41 | 1.0 | 1.0, 1.1 |
| >90 | ≥11.0 | 1,176 | 463 | 39 | 1.0 | 0.9, 1.1 |
| Underweight | ||||||
| 1–50 | 0–6.9 | 200 | 67 | 34 | 1 | Reference |
| 51–90 | 7.0–10.0 | 158 | 62 | 39 | 1.2 | 0.9, 1.6 |
| >90 | ≥10.1 | 39 | 21 | 54 | 1.5* | 1.0, 2.1 |
| Normal weight | ||||||
| 1–50 | 0–7.0 | 4,347 | 1,676 | 39 | 1 | Reference |
| 51–90 | 7.1–10.9 | 2,682 | 1,044 | 39 | 1.0 | 0.9, 1.1 |
| >90 | ≥11.0 | 790 | 304 | 39 | 1.0 | 0.9, 1.2 |
| Overweight | ||||||
| 1–50 | 0–6.5 | 1,598 | 721 | 45 | 1 | Reference |
| 51–90 | 6.6–11.0 | 1,308 | 581 | 44 | 1.0 | 0.9, 1.1 |
| >90 | ≥11.1 | 269 | 111 | 41 | 1.0 | 0.8, 1.1 |
* P < 0.05.
Weight (kg)/height (m)2. Prepregnancy body mass index was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
Adjustment was made for age.
A high weight gain during pregnancy weeks 15–30 was not associated with an increased incidence of UI in week 30 of pregnancy among all women (Table 2). This was also found for normal-weight and overweight women. However, there was a statistically significant association between weight gain greater than the 90th percentile (≥10.1 kg) and incident UI (RR = 1.5) among underweight women.
UI 6 months postpartum
High weight gain during weeks 0–15, 15–30, 30–delivery, or 0–delivery was generally not associated with increased UI prevalence 6 months postpartum in analysis of all women (Table 3). The same was found for normal and overweight women, while there was a trend among underweight women towards higher prevalence of UI with high weight gain.
Table 3.
Relative Risk of Urinary Incontinence Among Primiparous Women 6 Months Postpartum, by Weight Gain at Various Times During and After Pregnancy, Norwegian Mother and Child Cohort Study, 1999–2006
| Body Mass Indexa Group and Percentile | Weight Gain, kg | Total No. | Urinary Incontinence | Adjusted Relative Risk | 95% Confidence Interval | |
| No. | % | |||||
| Week 0–15b | ||||||
| All women | ||||||
| 1–50 | 0–3 | 6,349 | 2,031 | 32 | 1 | Reference |
| 51–90 | 4–6 | 3,115 | 965 | 31 | 1.0 | 0.9, 1.1 |
| >90 | ≥7 | 1,039 | 334 | 32 | 1.0 | 0.8, 1.1 |
| Underweight | ||||||
| 1–50 | 0–3 | 217 | 51 | 24 | 1 | Reference |
| 51–90 | 4–8 | 155 | 44 | 28 | 1.2 | 0.9, 1.7 |
| >90 | ≥9 | 27 | 8 | 30 | 1.2 | 0.7, 2.2 |
| Normal weight | ||||||
| 1–50 | 0–3 | 4,442 | 1,362 | 31 | 1 | Reference |
| 51–90 | 4–6 | 2,255 | 657 | 29 | 1.0 | 0.9, 1.0 |
| >90 | ≥7 | 688 | 211 | 31 | 0.9 | 0.8, 1.1 |
| Overweight | ||||||
| 1–50 | 0–3 | 1,659 | 606 | 37 | 1 | Reference |
| 51–90 | 4–7 | 837 | 308 | 37 | 1.0 | 0.9, 1.1 |
| >90 | ≥8 | 172 | 63 | 37 | 1.0 | 0.9, 1.2 |
| Week 15–30b | ||||||
| All women | ||||||
| 1–50 | 0–7.0 | 6,461 | 2,052 | 32 | 1 | Reference |
| 51–90 | 7.1–10.9 | 3,758 | 1,214 | 31 | 1.0 | 0.9, 1.1 |
| >90 | ≥11.0 | 1,155 | 357 | 32 | 1.0 | 0.9, 1.2 |
| Underweight | ||||||
| 1–50 | 0–6.9 | 197 | 52 | 26 | 1 | Reference |
| 51–90 | 7.0–10.0 | 155 | 37 | 24 | 0.9 | 0.7, 1.3 |
| >90 | ≥10.1 | 39 | 12 | 31 | 1.1 | 0.6, 1.8 |
| Normal weight | ||||||
| 1–50 | 0–7.0 | 4,299 | 1,279 | 30 | 1 | Reference |
| 51–90 | 7.1–10.9 | 2,645 | 834 | 32 | 1.1* | 1.0, 1.2 |
| >90 | ≥11.0 | 779 | 219 | 28 | 1.0 | 0.9, 1.2 |
| Overweight | ||||||
| 1–50 | 0–6.5 | 1,578 | 591 | 38 | 1 | Reference |
| 51–90 | 6.6–11.0 | 1,290 | 458 | 36 | 1.0 | 0.9, 1.1 |
| >90 | ≥11.1 | 261 | 96 | 37 | 1.0 | 0.9, 1.2 |
| Week 30–deliveryc | ||||||
| All women | ||||||
| 1–50 | 0–6.0 | 6,023 | 1,876 | 31 | 1 | Reference |
| 51–90 | 6.1–11.0 | 4,272 | 1,402 | 33 | 1.1 | 1.0, 1.1 |
| >90 | ≥11.1 | 930 | 289 | 31 | 1.0 | 0.9, 1.1 |
| Underweight | ||||||
| 1–50 | 0–5.6 | 19 | 44 | 23 | 1 | Reference |
| 51–90 | 5.7–10.0 | 152 | 42 | 28 | 1.1 | 0.8, 1.6 |
| >90 | ≥10.1 | 31 | 11 | 36 | 1.6 | 1.0, 2.6 |
| Normal weight | ||||||
| 1–50 | 0–6.0 | 4,138 | 1,246 | 30 | 1 | Reference |
| 51–90 | 6.1–10.5 | 2,641 | 799 | 30 | 1.0 | 0.9, 1.1 |
| >90 | ≥10.6 | 708 | 203 | 29 | 1.0 | 0.9, 1.1 |
| Overweight | ||||||
| 1–50 | 0–6.5 | 1,561 | 545 | 35 | 1 | Reference |
| 51–90 | 6.6–11.5 | 1,155 | 442 | 38 | 1.1 | 1.0, 1.2 |
| >90 | ≥11.6 | 295 | 113 | 38 | 1.1 | 0.9, 1.3 |
| Week 0–deliveryd | ||||||
| All women | ||||||
| 1–50 | 0–15.0 | 5,959 | 1,886 | 32 | 1 | Reference |
| 51–90 | 15.1–23.0 | 4,488 | 1,445 | 32 | 1.0 | 0.9, 1.1 |
| >90 | ≥23.1 | 1,148 | 346 | 30 | 1.0 | 0.8, 1.1 |
| Underweight | ||||||
| 1–50 | 0–15.0 | 202 | 50 | 25 | 1 | Reference |
| 51–90 | 15.1–24.0 | 162 | 44 | 27 | 1.1 | 0.8, 1.5 |
| >90 | ≥24.1 | 37 | 11 | 30 | 1.3 | 0.8, 2.2 |
| Normal weight | ||||||
| 1–50 | 0–15.0 | 4,041 | 1,212 | 30 | 1 | Reference |
| 51–90 | 15.1–23.0 | 3,160 | 947 | 31 | 1.0 | 1.0, 1.1 |
| >90 | ≥23.1 | 742 | 203 | 27 | 1.0 | 0.9, 1.1 |
| Overweight | ||||||
| 1–50 | 0–15.0 | 1,676 | 607 | 36 | 1 | Reference |
| 51–90 | 15.1–24.0 | 1,201 | 437 | 36 | 1.0 | 0.9, 1.1 |
| >90 | ≥24.1 | 303 | 112 | 37 | 1.0 | 0.9, 1.2 |
* P < 0.05.
Weight (kg)/height (m)2. Prepregnancy body mass index was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
Adjustment was made for age and mode of delivery.
Adjustment was made for age and birth weight.
Adjustment was made for age, mode of delivery, and birth weight.
In contrast, high total weight gain from the start of pregnancy to 6 months postpartum was more strongly associated with having UI than was high weight gain in any single subperiod in analyses of all women (Table 4). Corresponding results were found for all BMI strata. In analyses of all women in the study group, each kilogram of weight gain increased the relative risk for UI by 2.3% (RR = 1.023, 95% CI: 1.02, 1.03). Analyses of all women with weight gain greater than the 90th percentile (≥7.1 kg) from week 0 of pregnancy to 6 months postpartum showed a significant association with UI (RR = 1.3). The same trend was found in the normal and overweight subgroups.
Table 4.
Relative Risk of Urinary Incontinence Among Primiparous Women 6 Months Postpartum, by Weight Gain From Week 0 of Pregnancy to 6 Months Postpartum, Norwegian Mother and Child Cohort Study, 1999–2006
| Body Mass Indexa Group and Percentile | Weight Gain, kg | Total No. | Urinary Incontinence | Adjusted Relative Riskb | 95% Confidence Interval | |
| No. | % | |||||
| All women | ||||||
| 1–50 | ≤1.0 | 6,546 | 1,929 | 30 | 1 | Reference |
| 51–90 | 1.1–7.0 | 4,070 | 1,383 | 34 | 1.2* | 1.1, 1.2 |
| >90 | ≥7.1 | 1,134 | 422 | 37 | 1.3* | 1.2, 1.4 |
| Underweight | ||||||
| 1–50 | ≤2.0 | 225 | 50 | 22 | 1 | Reference |
| 51–90 | 2.1–8.0 | 144 | 44 | 31 | 1.4 | 1.0, 2.0 |
| >90 | ≥8.1 | 37 | 11 | 30 | 1.2 | 0.7, 2.1 |
| Normal weight | ||||||
| 1–50 | ≤1.0 | 4,501 | 1,235 | 27 | 1 | Reference |
| 51–90 | 1.1–7.0 | 2,875 | 967 | 34 | 1.2* | 1.2, 1.3 |
| >90 | ≥7.1 | 650 | 221 | 34 | 1.3* | 1.2, 1.5 |
| Overweight | ||||||
| 1–50 | ≤0.0 | 1,642 | 568 | 35 | 1 | Reference |
| 51–90 | 0.1–9.0 | 1,334 | 484 | 36 | 1.0 | 1.0, 1.1 |
| >90 | ≥9.1 | 279 | 128 | 46 | 1.4* | 1.2, 1.6 |
* P < 0.05.
Weight (kg)/height (m)2. Prepregnancy body mass index was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
Adjustment was made for age and mode of delivery.
From delivery to 6 months postpartum, there was a clear association between weight loss and lower prevalence of UI among all women who were incontinent during pregnancy, as well as in stratified analyses (Table 5). For each kilogram of weight loss among all women who were incontinent during pregnancy, the relative risk for UI decreased 2.1% (RR = 0.979, 95% CI: 0.97, 0.99). Weight loss greater than the 50th percentile (≥14.1 kg) was significantly associated with a lower prevalence of UI 6 months postpartum. Corresponding associations were found in stratified analyses among normal-weight and overweight women (Table 5). From delivery to 6 months postpartum, there was a significant association between weight loss greater than the 50th percentile (≥14.1 kg) and reduced risk of incident UI in analyses of all women who were continent during pregnancy. A corresponding association was found among normal-weight women (Table 5).
Table 5.
Relative Risk of Urinary Incontinence 6 Months Postpartum Among Primiparous Women, by Continence Status During Pregnancy and by Weight Loss From Delivery to 6 Months Postpartum, Norwegian Mother and Child Cohort Study, 1999–2006
| Body Mass Index Groupaand Percentile | Weight Loss, kg | Incontinent During Pregnancy | Continent During Pregnancy | ||||||||
| Total No. | UI Prevalence | Adjusted RRb | 95% CI | Total No. | UI Incidence | Adjusted RRb | 95% CI | ||||
| No. | % | No. | % | ||||||||
| All women | |||||||||||
| 1–50 | 0–14.0 | 2,520 | 1,283 | 51 | 1 | Reference | 3,549 | 815 | 23 | 1 | Reference |
| 51–90 | 14.1–21.0 | 1,807 | 825 | 46 | 0.9* | 0.8, 0.9 | 2,882 | 549 | 19 | 0.8* | 0.7, 0.9 |
| >90 | ≥21.1 | 419 | 157 | 38 | 0.7* | 0.6, 0.8 | 643 | 118 | 18 | 0.7* | 0.5, 0.8 |
| Underweight | |||||||||||
| 1–50 | 0–14.0 | 92 | 35 | 38 | 1 | Reference | 153 | 31 | 20 | 1 | Reference |
| 51–90 | 14.1–19.9 | 45 | 16 | 36 | 1.0 | 0.6, 1.5 | 75 | 11 | 15 | 0.7 | 0.4, 1.4 |
| >90 | ≥20.0 | 15 | 4 | 27 | 0.8 | 0.3, 1.9 | 18 | 6 | 33 | 1.6 | 0.8, 3.4 |
| Normal weight | |||||||||||
| 1–50 | 0–14.0 | 1,601 | 790 | 49 | 1 | Reference | 2,388 | 523 | 22 | 1 | Reference |
| 51–90 | 14.1–21.0 | 1,204 | 532 | 44 | 0.9* | 0.8, 0.9 | 2,061 | 375 | 18 | 0.9* | 0.8, 0.9 |
| >90 | ≥21.1 | 239 | 88 | 37 | 0.7* | 0.6, 0.8 | 385 | 65 | 17 | 0.7* | 0.7, 0.8 |
| Overweight | |||||||||||
| 1–50 | 0–14.0 | 734 | 410 | 56 | 1 | Reference | 666 | 168 | 25 | 1 | Reference |
| 51–90 | 14.1–22.0 | 531 | 261 | 49 | 0.9* | 0.8, 1.0 | 536 | 113 | 21 | 0.9* | 0.8, 0.9 |
| >90 | ≥22.1 | 127 | 53 | 42 | 0.8* | 0.6, 1.0 | 127 | 19 | 15 | 0.6* | 0.5, 0.8 |
Abbreviations: CI, confidence interval; RR, relative risk; UI, urinary incontinence.
* P < 0.05.
Weight (kg)/height (m)2. Prepregnancy body mass index was categorized into 3 groups according to World Health Organization recommendations: <18.5 (underweight), 18.5–24.9 (normal weight), and ≥25 (overweight and obese).
Adjustment was made for age, birth weight, and mode of delivery.
DISCUSSION
In this large cohort of primiparous women who were continent before pregnancy, we found that a high weight gain during pregnancy generally was not associated with UI 6 months postpartum. High weight gain from the start of pregnancy through 6 months postpartum was the only systematic weight-associated risk factor for UI 6 months postpartum. Weight loss after delivery was associated with decreased incidence of UI and increased remission of UI 6 months postpartum.
Our study design was an appropriate model for isolating the effect of weight gain and weight loss on UI in a first pregnancy. The nulliparous continent pelvis represents the best available clinical model of the unexposed pelvis (28). A major strength of the results obtained in this large cohort is their high precision, represented by narrow confidence intervals. We investigated how weight gain beyond the median level affects UI; but to isolate the effects of extreme weight gain, we also analyzed weight gain beyond the 90th percentile. Adjustments for relevant confounders in our study made the results more robust (27). We investigated several potential confounders. Adjustment for maternal age produced a small reduction in the relative risk, while adjustment for birth weight and mode of delivery produced a small increase in relative risk. Relevant adjustment was assessed for each exposure period and its related outcome, since confounders varied according to the time of the weight gain and the time of the reported UI.
A weakness in MoBa was the somewhat low inclusion rate. However, the overall MoBa data set is valid for risk estimates (22). There are only minor differences in demographic data and pregnancy-related variables when MoBa participants are compared with the total population of pregnant women in Norway (21). Among women who answered the questionnaire in week 30 of pregnancy, 87% completed the questionnaire 6 months postpartum (21). Weight before pregnancy and at delivery was reported retrospectively. However, 1 study indicated high correlation between recall of prepregnancy weight and actual prepregnancy weight, even many years after delivery (29). In addition, 6 months postpartum, there is high correlation between self-reported weight and documented weight during pregnancy (30). All Norwegian women have a pregnancy chart documenting weight before pregnancy and at the time of birth. Hence, data for weight at these 2 time points were easy for women to recollect. In general, the strengths of the associations were modest. We explored potential confounders and adjusted appropriately, but we cannot rule out residual confounding. Another limitation of our study is that women in MoBa reported UI status at week 30 and not at the time of delivery. Hence, we did not have information about the incidence of UI from week 30 to delivery, and consequently we could not investigate the association between weight gain and UI at the time of delivery. Therefore, we suggest that it is safe to assume that the incidence of UI during pregnancy is underreported. We elaborated more on the strengths and weaknesses apparent in MoBa and our study population in a previous report (23).
Among 10 previously published studies on UI and weight gain during pregnancy (10–19), none was designed to investigate this issue. They all had methodological weaknesses. None of those researchers stratified for BMI or clarified their categorization of weight gain when investigating the effect of weight gain during pregnancy on UI. Our study was conducted to overcome some of the limitations of these previous studies.
High weight gain during weeks 0–15 of pregnancy was associated with UI at week 30 of pregnancy, while high weight gain during weeks 15–30 showed no such association. Other investigators have also found nonsignificant associations between total weight gain during pregnancy and UI before delivery (14, 16, 18). In only 1 of these papers did the researchers present adjusted analyses (18). We have not found studies investigating the association between weight gain and UI during different trimesters.
The relative risks of UI after weight gain were rather similar for the total group of women and the subgroups of normal-weight and overweight women. However, weight gain among underweight women led to the highest relative risks and the largest absolute incidence and prevalence increases in UI. Underweight women had a large relative weight gain in comparison with other BMI groups, and this may explain our findings. However, when analyzing comparable percentwise increases in body weight, the significant associations between weight gain and UI during the different investigated time periods reappeared. Among underweight women, there was no association between weight loss postpartum and UI 6 months postpartum.
High weight gain during pregnancy did not affect UI 6 months postpartum. Hence, the association between UI during pregnancy and high weight gain during weeks 0–15 is less likely to be clinically important. This is in accordance with findings from other studies. Eason et al. (18) found no increased risk of UI 3 months postpartum among women who gained more than 17 kg during pregnancy as compared with women who gained less than 11 kg. In a Spanish study, Diez-Itza (19) found no association between total weight gain during pregnancy and persistent UI 1 year postpartum. However, our results contrast with those of a small Italian study investigating the association between weight gain and incontinence during the early postpartum period (10). We reanalyzed the data in the Italian study (10) and found an unadjusted relative risk of 2.0 for UI 3 weeks postpartum among women who gained ≥15 kg during pregnancy. These authors did not present stratified data for prepregnancy BMI, nor did they clarify their use of weight groups (10, 18, 19). Even though there is no consistent evidence for associations between weight gain during pregnancy and UI, several studies of nonpregnant women have found strong associations between weight gain and UI. In the Nurses' Health Study, Townsend et al. (8) reported an odds ratio of 3.5 for frequent UI in a general population of nonpregnant women who gained more than 20 kg over a 4-year time period.
There were significant associations between high weight gain from before pregnancy through 6 months postpartum and having UI 6 months postpartum. This association adds to the literature on the consistent association between BMI and UI (7). We also found decreased risk of UI 6 months postpartum by weight loss from the time of delivery to 6 months postpartum among women who were incontinent during pregnancy. Other studies of nonpregnant women have found a similar association with weight loss as our study; in a randomized controlled trial, Subak et al. (20) reported that 7% of overweight women with frequent UI became urinarily continent after a mean weight loss of 7.8 kg over a 6-month period. We believe that the associations between UI and weight loss postpartum in our study are of clinical importance.
Both pregnancy (31) and high BMI (7, 32) are established risk factors for UI. It has been suggested that weight gain during pregnancy partly explains the increased risk of UI both during pregnancy and in the postpartum period (33). The results of this purposely designed epidemiologic study do not support this. We also question whether weight gain per se leads to UI.
The association between weight gain and UI among nonpregnant women is primarily linked to adiposity (8). Weight gain in pregnancy is induced mainly by the growing fetus and pregnancy-related organs. This may explain why a 20-kg weight gain after 9 months does not necessarily increase the incidence of UI (14, 16, 18), as also found in our data, while a much smaller weight gain under nonpregnant conditions over a period of 1–2 years is associated with UI (8). This important difference indicates that there are factors that do not act similarly in pregnant and nonpregnant women. The location of the weight gained may play an important role (34, 35). Other factors might include the duration and speed of weight gain (36). Hormonal changes occurring during pregnancy, such as high levels of relaxin, may protect against the effects of weight gain (14), while hormonal changes associated with adiposity may not. In summary, our findings suggest that weight gain per se during pregnancy was not significantly associated with increased risk of UI associated with pregnancy.
Continence promotion postpartum mainly focuses on pelvic floor training. Our findings indicate that weight loss should be addressed in continence promotion postpartum to regain continence and reduce risk of UI. Weight loss postpartum, together with pelvic floor muscle training (37–39), may decrease the prevalence of UI in women postpartum. This may have clinically important implications.
In conclusion, our findings showed that weight gain during pregnancy was of little relevance to continence status during pregnancy and postpartum, and therefore could not explain the high incidence of UI during pregnancy. Weight gain from the start of pregnancy to 6 months postpartum was associated with increased prevalence of UI 6 months postpartum. Weight loss after delivery was associated with decreased incidence and increased remission of UI 6 months postpartum and thus should be addressed during continence promotion.
Supplementary Material
Acknowledgments
Author affiliations: Department of Public Health and Primary Health Care, University of Bergen, Bergen, Norway (Stian Langeland Wesnes, Steinar Hunskaar, Guri Rortveit); Department of Sports Medicine, Norwegian School of Sports Sciences, Oslo, Norway (Kari Bo); and Research Unit for General Practice, Uni Health, Bergen, Norway (Guri Rortveit).
Dr. Stian L. Wesnes' work on this study was supported by a grant from the Western Norway Regional Health Authority.
The Norwegian Mother and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health, the US National Institute of Environmental Health Sciences (grant N01-ES-85433), the US National Institute of Neurological Disorders and Stroke (grant 1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE Programme (grant 151918/S10).
The authors thank the MoBa investigators and the staff of the Medical Birth Registry of Norway for access to necessary data.
Preliminary results of this study were presented at the annual conference of the International Continence Society in San Francisco, California, on October 2, 2009.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- MoBa
Norwegian Mother and Child Cohort Study
- RR
relative risk
- UI
urinary incontinence
References
- 1.Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93(3):324–330. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 2.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. J Clin Epidemiol. 2000;53(11):1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 3.Hampel C, Wienhold D, Benken N, et al. Prevalence and natural history of female incontinence. Eur Urol. 1997;32(suppl 2):3–12. [PubMed] [Google Scholar]
- 4.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarelli P, Brown WJ. Leaking urine in Australian women: prevalence and associated conditions. Women Health. 1999;29(1):1–13. doi: 10.1300/J013v29n01_01. [DOI] [PubMed] [Google Scholar]
- 6.Rortveit G, Daltveit AK, Hannestad YS, et al. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348(10):900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 7.Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourol Urodyn. 2008;27(8):749–757. doi: 10.1002/nau.20635. [DOI] [PubMed] [Google Scholar]
- 8.Townsend MK, Danforth KN, Rosner B, et al. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110(2):346–353. doi: 10.1097/01.AOG.0000270121.15510.57. [DOI] [PubMed] [Google Scholar]
- 9.Waetjen LE, Feng WY, Ye J, et al. Factors associated with worsening and improving urinary incontinence across the menopausal transition. Obstet Gynecol. 2008;111(3):667–677. doi: 10.1097/AOG.0b013e31816386ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alfonso A, Iovenitti P, Carta G. Urinary disorders during pregnancy and postpartum: our experience. Clin Exp Obstet Gynecol. 2006;33(1):23–25. [PubMed] [Google Scholar]
- 11.Troiano L, Pregazzi R, Bortoli P, et al. Urinary disorders in the puerperium. Study of the major risk factors [in Italian] Minerva Ginecol. 2000;52(7-8):289–297. [PubMed] [Google Scholar]
- 12.Glazener CM, Herbison GP, MacArthur C, et al. New postnatal urinary incontinence: obstetric and other risk factors in primiparae. BJOG. 2006;113(2):208–217. doi: 10.1111/j.1471-0528.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiarelli P, Campbell E. Incontinence during pregnancy. Prevalence and opportunities for continence promotion. Aust N Z J Obstet Gynaecol. 1997;37(1):66–73. doi: 10.1111/j.1479-828x.1997.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 14.Kristiansson P, Samuelsson E, von Schoultz B, et al. Reproductive hormones and stress urinary incontinence in pregnancy. Acta Obstet Gynecol Scand. 2001;80(12):1125–1130. doi: 10.1034/j.1600-0412.2001.801209.x. [DOI] [PubMed] [Google Scholar]
- 15.Pregazzi R, Sartore A, Troiano L, et al. Postpartum urinary symptoms: prevalence and risk factors. Eur J Obstet Gynecol Reprod Biol. 2002;103(2):179–182. doi: 10.1016/s0301-2115(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 16.Sottner O, Zahumensky J, Krcmar M, et al. Urinary incontinence in a group of primiparous women in the Czech Republic. Gynecol Obstet Invest. 2006;62(1):33–37. doi: 10.1159/000091820. [DOI] [PubMed] [Google Scholar]
- 17.van Brummen HJ, Bruinse HW, van de Pol G, et al. The effect of vaginal and cesarean delivery on lower urinary tract symptoms: what makes the difference? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(2):133–139. doi: 10.1007/s00192-006-0119-5. [DOI] [PubMed] [Google Scholar]
- 18.Eason E, Labrecque M, Marcoux S, et al. Effects of carrying a pregnancy and of method of delivery on urinary incontinence: a prospective cohort study. BMC Pregnancy Childbirth. 2004;4(1):4. doi: 10.1186/1471-2393-4-4. (doi: 10.1186/1471-2393-4-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diez-Itza I, Ibañez L, Arrue M, et al. Influence of maternal weight on the new onset of stress urinary incontinence in pregnant women. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(10):1259–1263. doi: 10.1007/s00192-009-0923-9. [DOI] [PubMed] [Google Scholar]
- 20.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 22.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 23.Wesnes SL, Hunskaar S, Bo K, et al. The effect of urinary incontinence status during pregnancy and delivery mode on incontinence postpartum. A cohort study. BJOG. 2009;116(5):700–707. doi: 10.1111/j.1471-0528.2008.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen KM, Abrams B, Bouchard C, et al. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: Institute of Medicine, National Academy of Sciences; 2009. [PubMed] [Google Scholar]
- 26.Breslow NE, Day NE. Statistical methods in cancer research. Vol 1. The analysis of case-control studies. IARC Sci Publ. 1980;(32):5–338. [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 28.Farrell SA, Allen VM, Baskett TF. Parturition and urinary incontinence in primiparas. Obstet Gynecol. 2001;97(3):350–356. doi: 10.1016/s0029-7844(00)01164-9. [DOI] [PubMed] [Google Scholar]
- 29.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- 30.Oliveira AF, Gadelha AM, Leal Mdo C, et al. Study of validity in self-reported weight and height among pregnant women treated at municipal maternity hospitals in Rio de Janeiro, Brazil [in Portuguese] Cad Saude Publica. 2004;20(suppl 1):S92–S100. doi: 10.1590/s0102-311x2004000700010. [DOI] [PubMed] [Google Scholar]
- 31.Wesnes SL, Rortveit G, Bø K, et al. Urinary incontinence during pregnancy. Obstet Gynecol. 2007;109(4):922–928. doi: 10.1097/01.AOG.0000257120.23260.00. [DOI] [PubMed] [Google Scholar]
- 32.Hannestad YS, Rortveit G, Daltveit AK, et al. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110(3):247–254. [PubMed] [Google Scholar]
- 33.Milsom I, Altman D, Lapitan MC, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP) In: Abrams P, Cardozo L, Khoury S, editors. Incontinence: 4th International Consultation on Incontinence. Plymouth, United Kingdom: Health Publications Ltd; 2009. pp. 37–111. [Google Scholar]
- 34.Han MO, Lee NY, Park HS. Abdominal obesity is associated with stress urinary incontinence in Korean women. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):35–39. doi: 10.1007/s00192-005-1356-8. [DOI] [PubMed] [Google Scholar]
- 35.Brown JS, Grady D, Ouslander JG, et al. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94(1):66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 36.Mishra GD, Hardy R, Cardozo L, et al. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes (Lond) 2008;32(9):1415–1422. doi: 10.1038/ijo.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay-Smith J, Mørkved S, Fairbrother KA, et al. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD007471. CD007471. (doi: 10.1002/14651858.CD007471) [DOI] [PubMed] [Google Scholar]
- 38.Mørkved S, Bø K. The effect of postpartum pelvic floor muscle exercise in the prevention and treatment of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(4):217–222. doi: 10.1007/BF02765817. [DOI] [PubMed] [Google Scholar]
- 39.Mørkved S, Bø K. Effect of postpartum pelvic floor muscle training in prevention and treatment of urinary incontinence: a one-year follow up. BJOG. 2000;107(8):1022–1028. doi: 10.1111/j.1471-0528.2000.tb10407.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.