Abstract
Purpose
The objectives of this study were to determine the effects of permeant lipophilicity on permeant uptake into and transport across human sclera for transscleral delivery.
Methods
Model permeants with a wide range of lipophilicities were selected and studied with human sclera. Uptake experiments were carried out to measure permeant partitioning into the sclera. Transport experiments were performed in side-by-side diffusion cells, and the permeability coefficients and transport lag times of the permeants across the sclera were evaluated.
Results
Permeants with higher lipophilicity showed higher partition coefficients to human sclera, and the apparent transport lag time also increased significantly as the permeant lipophilicity increased. No correlation between the permeability coefficients and lipophilicity of the model permeants was observed in this study with human sclera. A hypothesis on the different findings between the present and previous studies was proposed.
Conclusions
Permeants with higher lipophilicity exhibited stronger binding to human sclera and would therefore lead to larger permeant partitioning to the sclera and longer transport lag time. The steady-state permeability coefficients of the permeants were not significantly affected by permeant lipophilicity.
Keywords: human sclera, lag time, lipophilicity, partition coefficient, permeability
INTRODUCTION
Vision loss due to posterior eye diseases, such as age-related macular degeneration, diabetic retinopathy, glaucoma, and retinitis pigmentosa, accounts for most of the irreversible blindness world-wide (1,2). However, effective drug delivery to the posterior segment of the eye still remains a challenge for treating these diseases. In many instances, the traditional topical route is ineffective in delivering and achieving therapeutic levels of drugs to the posterior tissues of eye (1,3). Systemic administration requires high doses of drugs and has the potential of causing systemic toxicities (1,2,4). Intravitreal injections are invasive, and complications such as retinal detachment, endophthalmitis, and cataract after injections have been reported, particularly after repeated injections in the treatment of chronic eye diseases (2,4). Due to these various problems, a safe, effective, and robust drug delivery method is needed.
With the relatively large area and high permeability of sclera (1,5), transscleral delivery was suggested to be a promising method to deliver drugs to the posterior segment of the eye. A number of approaches, including transscleral iontophoresis (5,6), episcleral exoplants (7), and fibrin sealants (8), have been studied for transscleral drug delivery. Although promising, further understanding on how drug physicochemical properties could influence the delivery is necessary to optimize transscleral drug delivery.
Previous studies have suggested the influence of drug lipophilicity on transscleral delivery. For example, the effects of solute lipophilicity on solute permeability of bovine and porcine eyes were studied by Cheruvu and Kompella, and the results indicated that the solute partition coefficients measured in bovine tissues increased with the logarithm of distribution coefficients of the solutes, while the solute permeability coefficients measured in both bovine and porcine tissues exhibited a negative correlation with the logarithm of distribution coefficients (4). Kadam and Kompella investigated the partitioning of eight β-blockers into bovine sclera and found that the lipophilic β-blockers had higher tissue partitioning than the hydrophilic ones (9). A literature review was carried out by Prausnitz and Noonan, and the results with bovine, rabbit, and human sclera showed that the sclera permeability was relatively independent of the permeant distribution coefficient (10). Data about human sclera are limited, and the influences of drug-to-human sclera binding on the uptake and transport behavior of drugs have not been systematically studied. Investigations on the relationship between drug lipophilicity and binding to human sclera and how the relationship would affect drug transport behavior across human sclera are therefore important.
In the present study, uptake and transport experiments were conducted with human cadaveric sclera using permeants with a wide range of lipophilicities. The objectives were to (a) examine the partition coefficients of permeants to human sclera, (b) analyze the relationship between permeant lipophilicity and partitioning, and (c) determine the influence of permeant lipophilicity on transscleral transport. A hypothesis that permeant lipophilicity mainly affected transient transscleral transport and not steady-state transport was also examined. The results in this study could help pharmaceutical researchers develop better transscleral drug delivery systems for treating eye diseases.
MATERIALS AND METHODS
Materials
Phosphate-buffered saline (PBS, pH 7.4, consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride, 0.137 M sodium chloride) was prepared by dissolving PBS tablets (Sigma-Aldrich, St. Louis, MO) in distilled deionized water and was preserved with 0.02% (w/v) sodium azide (NaN3). Sodium azide at purity ≥98% was purchased from Acros Organics (Morris Plains, NJ). Ethanol (anhydrous ethyl alcohol) was purchased from Fisher Scientific (Rochester, NY). 14C-mannitol (1-14C, 51.0 mCi/mmol), 14C-tetraethylammonium bromide (1-14C, 3.5 mCi/mmol), 14C-salicylic acid (7-14C, 47.0 mCi/mmol), 3H-dexamethasone (6,7-3H(N)-, 23.1 Ci/mmol) and 3H-corticosterone (1,2,6,7-3H(N)-, 70.0 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). 14C-urea (55 mCi/mmol), 3H-estradiol (2,4-3H, 24.5 Ci/mmol), 3H-atenolol (ring-3H, 5.1 Ci/mmol), 3H-fluconazole (2.5 Ci/mmol), and 3H-paclitaxel (benzamido-2,6-3H(N)-, 45.1 Ci/mmol) were purchased from Moravek Biochemicals and Radiochemicals (Brea, CA). 3H-cyclosporin A (mebmt-β-3H, 10 Ci/mmol) was obtained from GE Healthcare Life Sciences, Amersham (Piscataway, NJ). 3H-metoprolol (O-methyl-3H, 80 Ci/mmol) was obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Urea was purchased from Acros Organics (Morris Plains, NJ). Atenolol at purity >99% and corticosterone at purity ≥92% were purchased from Sigma-Aldrich (St. Louis, MO). Estradiol (USP grade) and cyclosporin A (USP grade) were obtained from Letco Medical (Decatur, AL). The physicochemical properties of the permeants are summarized in Table I.
Table I.
Physicochemical Properties of the Permeants
| Permeant | Log Doct | Molecular Weight (g/mol) |
Molecular Radius (nm) a |
pKa |
|---|---|---|---|---|
| Mannitol | −2.9b, d | 182 | 0.42 | |
| Tetraethylammonium | −2.8c, e | 130 | 0.37 | |
| Urea | −2.1c, f | 60 | 0.29 | |
| Salicylate | −1.4b, f | 138 | 0.38 | 2.9l |
| Atenolol | −1.4b, g | 266 | 0.47 | 9.3g |
| Metoprolol | 0.2b, g | 267 | 0.47 | 9.6g |
| Fluconazole | 0.5b, h | 306 | 0.50 | 2.0m |
| Dexamethasone | 1.8c, f | 392 | 0.54 | |
| Corticosterone | 1.9c, f | 346 | 0.52 | |
| Estradiol | 2.3b, i | 272 | 0.48 | |
| Cyclosporin A | 4.3b, j | 1203 | 0.78 | |
| Paclitaxel | 6.0c, k | 854 | 0.70 |
The molecular radius (r) was estimated by , where MW is the molecular weight, NAV is the Avagadro’s number, and ρ is the density of the molecule. In this study, the densities of the molecules were assumed to be 1 g/cm3;
Octanol-pH 7.4 buffer distribution coefficient;
Octanol-water distribution coefficient;
(4);
(25);
Experimental value obtained from the Estimation Programs Interface (EPI) database;
(9);
(26);
Log Doct values of estradiol vary in the literature from 2.29 to 3.94, and a value of 2.3 (27) was used in the present study;
(28);
(29);
(30);
(31)
Preparation of the Sclera
Cadaver eyes were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA) and Moran Eye Center at the University of Utah (Salt Lake City, UT). The age of the human donors ranged from 55–75, and the causes of death included sepsis, cardiopulomonary arrest, intracranial hematoma, and lung cancer, without any known eye diseases. The tissues were stored in moisture chambers at 4°C. Before uptake and transport experiments, the tissues were cleaned at room temperature, and the adhering tissues, including retina and choroid, were removed from the sclera. The sclera was then equilibrated in PBS at room temperature and cut into pieces for the uptake and transport experiments. The thickness of the hydrated sclera was measured using a micrometer (Mitutoyo, Kawasaki, Kanagawa, Japan). The sclera used in the present study had thickness ranging from 0.7 to 1.0 mm. The use of human tissues was approved by the Institutional Review Board at the University of Cincinnati (Cincinnati, OH).
Uptake Experiment
After hydration, the sclera of about 0.5×0.5 cm2 was removed from PBS, blotted with Kimwipes® to remove the solution on the sclera surfaces, and quickly weighed (i.e., wet weight). The sclera was then dried at room temperature overnight to a constant weight (i.e., dry weight). For the sclera in the present study, the wet weight was 0.067±0.013 g and the dry weight was 0.018±0.003 g (mean ± SD, n=20). The water content of the sclera was about 73% of the wet weight, which is consistent with the range of 65–75% reported in the literature (11).
To determine permeant uptake into the sclera, the dry sclera was equilibrated with the equilibration solution for 24 h at room temperature. The equilibration solutions were 10 mL PBS mixed with 20 µL of 14C-mannitol, 14C-tetraethylammonium, 14C-urea, or 14C-salicylic acid stock solutions, or 2 µL of 3H-dexamethasone, 3H-corticosterone, 3H-estradiol, 3H-atenolol, 3H-metoprolol, 3H-fluconazole, 3H-cyclosporin A, or 3H-paclitaxel stock solutions. After equilibration, the sclera was removed from the equilibration solution, blotted with Kimwipes®, and placed in a vial for extraction. Successive 24-h extractions were performed with the sclera in 1 mL PBS. After each 24 h of extraction, the tissue was removed from the vial with a pair of forceps and placed in a new vial; the procedure was repeated with fresh PBS. The extraction solutions in the vials were mixed with 10 mL liquid scintillation cocktail (Ultima Gold™, PerkinElmer Life and Analytical Sciences, Shelton, CT), and the radioactivity was analyzed by a liquid scintillation counter (Beckman Coulter LS 6500, Fullerton, CA). Twenty microliters of the equilibration solution were also taken after removal of the sclera sample from the equilibration solution, and mixed with 980 µL of PBS and 10 mL of the liquid scintillation cocktail for liquid scintillation counting. For the uptake studies of the lipophilic permeants 3H-estradiol, 3H-cyclosporin A, and 3H-paclitaxel, a final extraction with 1 mL 50% (v/v) ethanol in PBS was conducted to ensure complete extraction. In the experiments, the binding of permeant to pipette tips was checked, and corrections were made in the calculations of the solution concentrations. The partition coefficient (Ksclera) was defined as the ratio of the apparent concentration of the permeant in the sclera to the concentration of the permeant in the equilibration solution:
| (1) |
where Mextraction is the total amount of permeant extracted from the sclera, Wwet is the wet weight of the sclera, and Cequilibration is the weight-weight concentration of the permeant in the equilibration solution assuming that the density of the solution is 1 g/mL. The effective porosity (εeff) was defined as the ratio of the effective volume accessible by the permeant in the sclera to the total volume of the hydrated sclera and calculated by
| (2) |
where ρ is the density of the hydrated sclera. The effective porosity measures the effective volume available for (or capacity of) permeant loading in the sclera. The density of the sclera was 1.3 ± 0.1 g/cm3 in the present study (mean ± SD, n=6), determined by dividing the wet weight of the sclera by the product of sclera surface area and thickness.
Transport Experiment
Five permeants, including urea, atenolol, corticosterone, estradiol, and cyclosporin A, were selected for the transport experiments using side-by-side diffusion cells. Donor solutions were prepared by mixing trace amounts of corresponding radiolabeled permeants into 2 µg/mL of non-radiolabeled permeants in PBS to provide the radioactivity of the donor solution in the range of 1×105–2×106 dpm/mL. Non-radiolabeled urea and atenolol solutions were prepared by directly dissolving appropriate amounts of the permeants in PBS. Non-radiolabeled corticosterone, estradiol, and cyclosporin A solutions were made by first dissolving the permeants in ethanol to get 5 mg/mL solutions and followed by dilution in PBS to obtain a final concentration of 2 µg/mL. Preliminary studies showed that the concentration of 2 µg/mL was below the solubilities of these permeants. PBS was used as the receiver solution.
Fully hydrated sclera of approximately 1.4×1.4 cm2 was sandwiched between the two half-cells of the side-by-side diffusion cells with an effective diffusion area of approximately 0.2 cm2 and with the episclera side facing the donor chamber. The diffusion cells were placed in a circulating water bath at 37 ± 2°C. The volume of donor and receiver solutions was 2 mL. The transport experiments were conducted under sink conditions with continuous stirring.
The durations of the transport experiments were at least 4 times greater than the transport lag time and were in the range of 2–15 h depending on the permeants studied. At predetermined time points, 10-µL and 1-mL samples were taken from the donor and receiver chambers, respectively, and mixed with 10 mL of the liquid scintillation cocktail for assay. After each sampling, fresh PBS of 1 mL was added to the receiver chamber to maintain a constant volume of the receiver solution. Donor solutions were also replaced at predetermined time intervals to ensure less than 10% depletion of the permeants in the donor solutions. The binding of permeants to pipette tips was checked, and corrections were made in the calculations of the permeant concentrations in the solutions. The amount of permeant transported across the sclera was determined by the radioactivity (in dpm) transported across the sclera and the specific activity (dpm/µg) of the radiolabeled permeant in the donor solution. The specific activity was determined by dividing the radioactivity of the permeant in the fresh donor solution by the concentration of the non-radiolabeled permeant in the solution.
The cumulative amount of a permeant transported across the sclera (Q) was plotted against time (t). The apparent or steady-state flux of the permeant (𝒥) was calculated from the slope of the linear regression of the linear region in the plot (ΔQ/Δt) divided by the effective diffusion area (A). The transport lag time (tlag) was calculated from the x-intercept of the linear regression line. The apparent or steady-state permeability coefficient (P) was calculated by dividing the flux by the concentration of the permeant in the donor (CD):
| (3) |
| (4) |
Statistical Analysis
The Student’s t-test with one-tailed distribution was employed to evaluate the statistical differences in the experiments. Differences were considered to be significant at a level of p<0.05. The means ± standard deviations (SD) of the data are presented.
Theory and Equations
In general, the steady-state permeability coefficient (P) is related to the effective diffusion coefficient (Deff) and effective partition coefficient (Keff) by
| (5) |
where h is the effective thickness of the membrane. The transport lag time (tlag) can be expressed by
| (6) |
For a porous membrane with aqueous transport pathways of effective pore size several orders of magnitude greater than the permeant size, such as the sclera (12) and the permeants used in the present study, when significant permeant-to-membrane binding occurs (or the permeant partitions into a membrane domain not involved in transport), Deff and Keff can be expressed by
| (7) |
| (8) |
where Daq is the free diffusion coefficient in the aqueous solution, K is the partition coefficient of the permeant from the aqueous solution into the membrane in the absence of binding, and R is the binding factor. Combining Eqs. 5–8, the steady-state permeability coefficient and transport lag time become
| (9) |
| (10) |
It should be emphasized that while the transport lag time increases with the extent of permeant binding to the membrane, the steady-state permeability is not affected by the binding. This phenomenon is consistent with the theory presented by Flynn et al. (13), i.e., the steady-state flux is unaffected by permeant binding, but the lag time is directly related to the binding.
RESULTS
Uptake Study
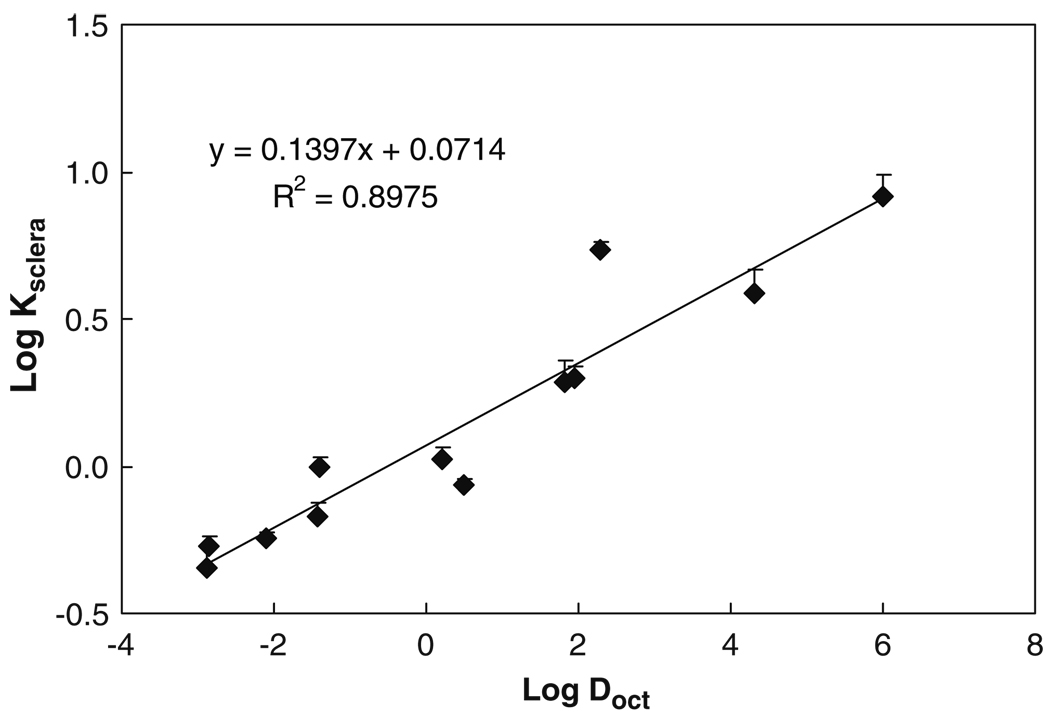
Fig. 1 presents the relationship between the sclera partition coefficient (Ksclera) and octanol-buffer (or octanol-water) distribution coefficient (Doct) of the permeants. In this study, Keff in Eq. 5 was approximated by Ksclera, which can be measured experimentally. The plot shows that lipophilic permeants exhibit higher partition coefficients than hydrophilic permeants. The partition coefficient of the most lipophilic paclitaxel in this study was about 18 times larger than the most hydrophilic mannitol. The partition coefficients increase linearly with the distribution coefficient of permeants with a slope of 0.14 in the plot of Log Ksclera vs Log Doct. This linear free energy relationship between sclera and octanol suggests the relatively polar nature of the sclera with lipophilicity significantly less than that of octanol.
Fig. 1.
Relationship between human sclera partition coefficient (Ksclera) and octanol-buffer (or octanol-water) distribution coefficient (Doct) of permeants. Data represent the mean and standard deviation of four sclera samples for each permeant.
Table II presents the effective porosity of human sclera for the permeants calculated using the uptake data and Eq. 2. The effective porosity determined by water uptake shown in the table was calculated using the water content and the density of the hydrated sclera. The results in the table show a trend of an increase in the effective porosity with the permeant lipophilicity. While the effective porosities determined by polar permeant uptake were typically less than that of water, the effective porosities determined by lipophilic permeant uptake were significantly higher than that of water. This indicates that the uptake of permeant into the sclera could be influenced by the lipophilicity of the permeant.
Table II.
Effective Porosities of the Human Sclera for the Permeants
| Permeant | Effective Porositya |
|---|---|
| Water | 0.93 ± 0.04 |
| Mannitol | 0.58 ± 0.08 |
| Tetraethylammonium | 0.68 ± 0.06 |
| Urea | 0.72 ± 0.04 |
| Salicylate | 0.87 ± 0.08 |
| Atenolol | 1.27 ± 0.09 |
| Metoprolol | 1.35 ± 0.13 |
| Fluconazole | 1.11 ± 0.04 |
| Dexamethasone | 2.5 ± 0.4 |
| Corticosterone | 2.6 ± 0.3 |
| Estradiol | 7.0 ± 0.4 |
| Cyclosporin A | 5.0 ± 1.0 |
| Paclitaxel | 10.6 ± 1.9 |
Data represent the mean and standard deviation of at least four sclera samples for each permeant.
The effective porosity determined by water was calculated using the water content and the density of sclera. The effective porosities determined by the permeants were calculated from their uptake studies using Eq. 2
Under the experimental condition of pH 7.4 and with similar lipophilicities and molecular sizes, positively charged atenolol (pKa 9.3 and Log Doct = −1.4) exhibited higher partition coefficient than negatively charged salicylate (pKa 2.9 and Log Doct = −1.4) (p<0.05). In like manner, the partition coefficient of positively charged metoprolol (pKa 9.6 and Log Doct = 0.2) was higher than the negatively charged fluconazole (pKa 2.0 and Log Doct = 0.5) (p<0.05) of similar lipophilicities and molecular sizes. The same pattern was also observed in the results of the effective porosity (Table II) where the effective porosity determined by atenolol was larger than that of salicylate and the effective porosity determined by metoprolol was larger than that of fluconazole. These results suggest that in addition to the effect of permeant lipophilicity, permeant-to-sclera charge-charge interaction also has an impact on permeant partitioning into the sclera. For negatively charged sclera at pH 7.4, the positively charged permeants generally show higher partition coefficients than the negatively charged permeants in the present study.
Transport Study
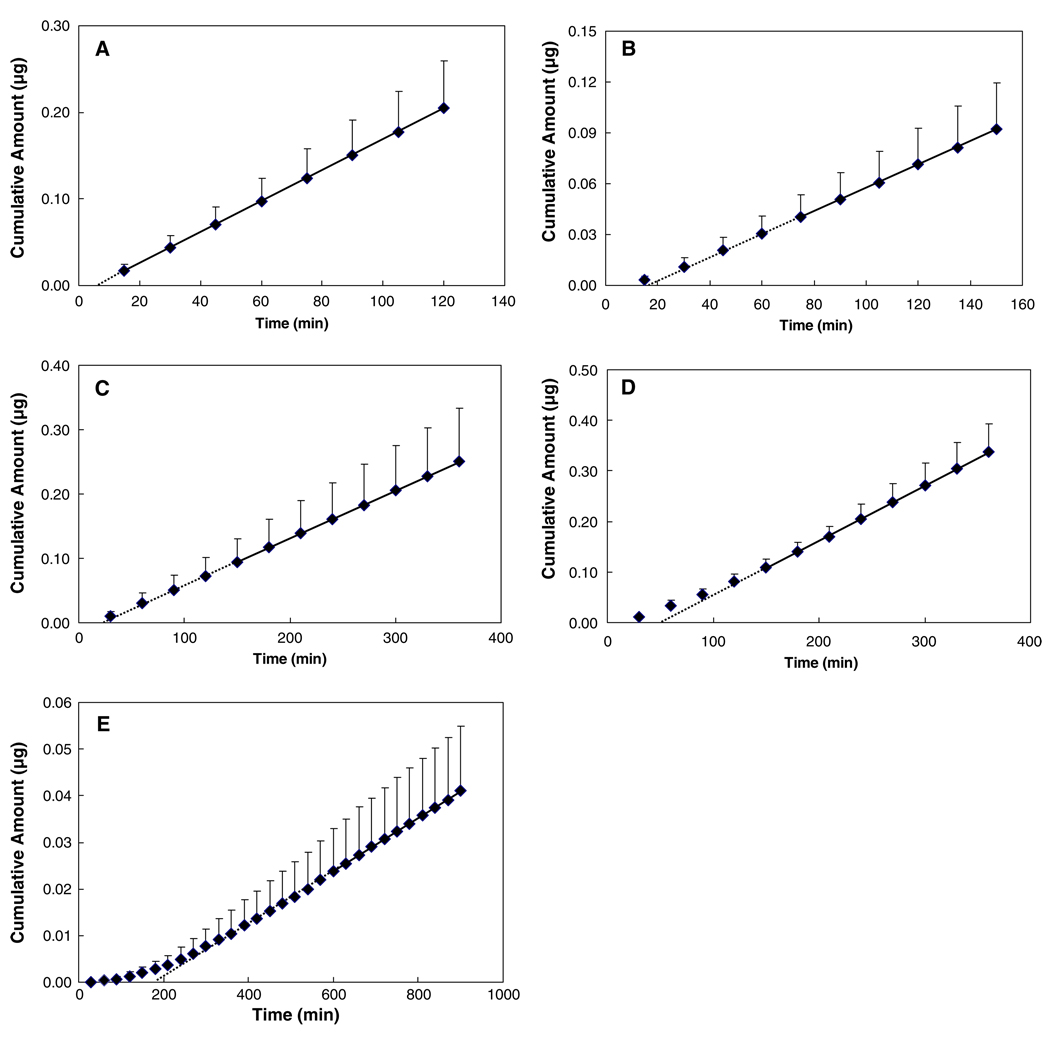
Urea, atenolol, corticosterone, estradiol, and cyclosporin A with the Log Doct ranging from −2.1 to 4.3 were selected as the model permeants in the transport experiments. The plots of the cumulative amounts of permeant transported across sclera versus time for these five permeants are shown in Fig. 2. These permeation profiles generally show good linear regression in the later stage of the transport experiments, suggesting that the steady-state condition has been attained. The results in the figure clearly illustrate that the lipophilic permeants required a longer time to reach steady state than the hydrophilic permeants in the transport study.
Fig. 2.
Plots of cumulative amounts of (A) urea, (B) atenolol, (C) corticosterone, (D) estradiol, and (E) cyclosporin A permeated through human sclera versus time. Data represent the mean and standard deviation of four sclera samples for each permeant. Solid lines show the linear portion of the permeation profiles and dotted lines show the x-intercept of the linear regression lines.
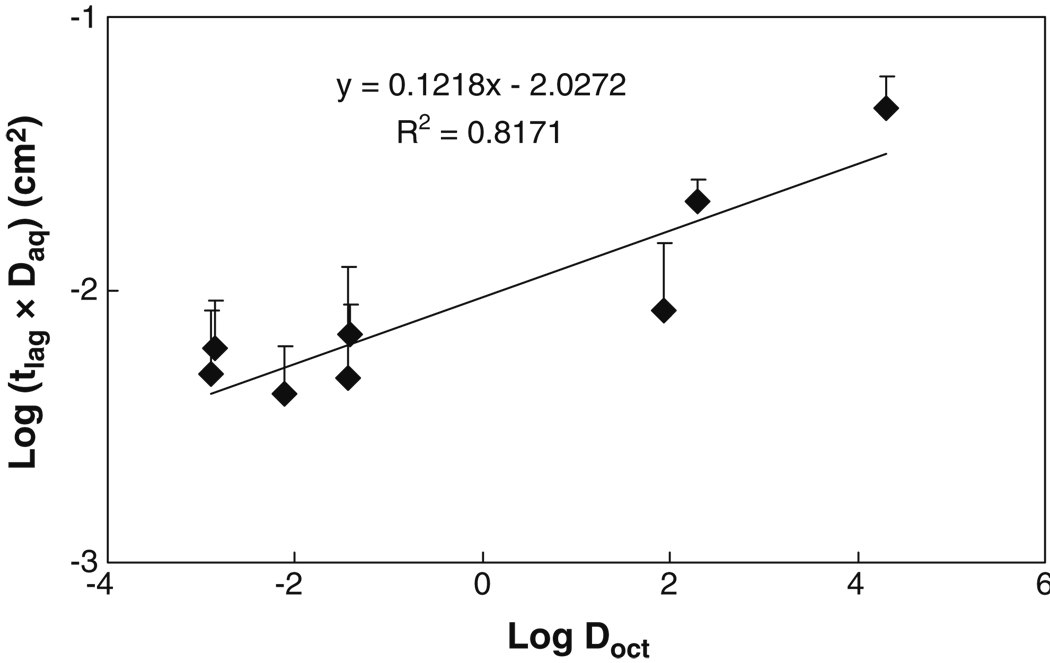
To delineate the effects of permeant lipophilicity and size on permeant transport, the transport lag times are normalized by the free diffusion coefficients in the aqueous solution (Daq), and the logarithm of the normalized lag time (tlag × Daq) was plotted against Log Doct (Fig. 3). The transport data of tetraethylammonium, salicylate, and mannitol from previous studies (12,14) are also included in the figure for the analyses. Fig. 3 shows that the lipophilic permeants exhibited significantly longer lag times than the hydrophilic permeants in the present transport study. The normalized lag times of lipophilic cyclosporin A and estradiol were about 20 and 6 times larger than that of urea, respectively. Fig. 3 also shows a linear regression line of the normalized lag time against permeant lipophilicity, which has a slope of approximately 0.12. This result demonstrates that higher permeant lipophilicity would lead to longer lag time in permeant transport across the sclera.
Fig. 3.
Relationship between transport lag time (tlag) in transport experiment through human sclera and permeant octanol-buffer (or octanol-water) distribution coefficient (Doct). The products of transport lag time and free aqueous diffusion coefficient (Daq) of permeants in water at 37°C are plotted against Log Doct. Free diffusion coefficients were calculated using the Stokes–Einstein equation, the molecular radii in Table I, and the viscosity of water at 37°C. Data represent the mean and the standard deviation of at least four sclera samples for each permeant. The lag time data of tetraethylammonium, salicylate, and mannitol are obtained from previous studies (12,14).
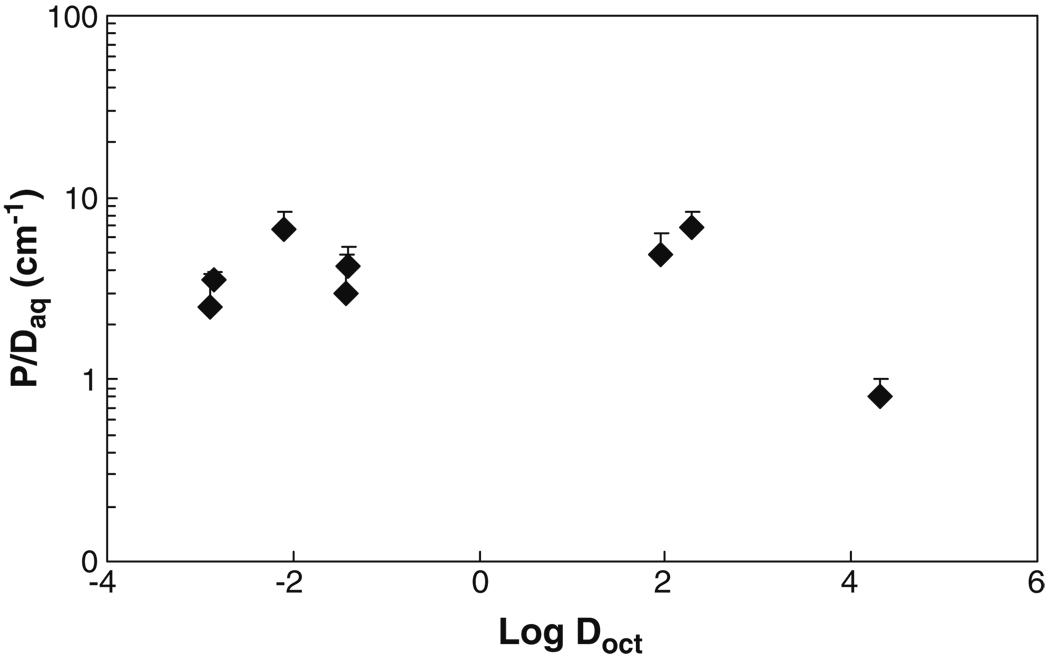
Fig. 4 shows the relationship between the permeability coefficient and the lipophilicity of the permeant. The permeability coefficients were normalized by the free diffusion coefficients of the permeants (P/Daq) to delineate the effect of permeant lipophilicity upon the sclera permeability and plotted against Log Doct in the figure. The transport data of tetraethylammonium, salicylate, and mannitol from our previous studies (12,14) are also included in the figure for the analyses. The normalized permeability coefficients for mannitol, tetraethylammonium, salicylate, urea, atenolol, corticosterone, and estradiol were essentially the same, independent of their lipophilicities. These results indicate that the steady-state permeability coefficients of human sclera for the permeants are not significantly affected by permeant lipophilicity under the condition in the present study. The lack of a correlation between permeability coefficient and permeant lipophilicity suggests an aqueous pore pathway transport mechanism rather than a lipid partitioning and diffusion transport mechanism. For cyclosporin A in Fig. 4, the normalized permeability coefficient is different from the other permeants. This may be due to experimental uncertainties related to the binding of the permeant to the apparatus in the transport experiments even though a number of extra steps were implemented in the study of this permeant, such as checking and correcting for the binding of cyclosporin A to pipette tips, replacing the donor solution frequently to maintain a relative constant concentration in the donor solution, and frequent sampling to maintain a steady-state condition after steady state was achieved. In addition to permeant lipophilicities, the results also show no significant difference between the steady-state permeability coefficients of human sclera for the positively charged, negatively charged, and neutral permeants under the experimental conditions in the present transport study.
Fig. 4.
Relationship between steady-state permeability coefficient (P) in transport experiment through human sclera and permeant octanol-buffer (or octanol-water) distribution coefficient (Doct). The ratios of the steady-state permeability to free aqueous diffusion coefficient (Daq) of permeants in water at 37°C are plotted against Log Doct. Data represent the mean and the standard deviation of at least four sclera samples for each permeant. The permeability coefficient data of tetraethylammonium, salicylate, and mannitol are obtained from previous studies (12,14).
It should be pointed out that the results in the present study are different from those in a previous study (4), which has concluded that the permeability in transscleral transport decreased with solute lipophilicity or the sclera was more permeable to negatively charged solutes than positively charged solutes. This inconsistency could be due to the different experimental designs in these studies and will be discussed in detail in the Discussion.
DISCUSSION
Influences of Permeant Lipophilicity on Permeant Sclera Partitioning and Transport
Sclera is a relatively porous tissue. The major component of the sclera is collagen, which comprises about 75% of dry weight of the sclera (1). At physiological pH of 7.4, human sclera is negatively charged (15). As a result, the mechanism of permeant uptake into and transport across the sclera can be described by an aqueous pore pathway model with permeant molecular size, charge, and lipophilicity as the major influencing factors.
The collagen and noncollagenous proteins in the sclera could act as the binding sites for permeants. The hypothesis of permeant-sclera binding during transport (rather than a lipid partitioning and diffusion transport mechanism) is supported by the observed increase in the partition coefficients of the permeants with the lipophilicities of the permeants (Fig. 1), the correlation between transport lag times and the lipophilicities of the permeants (Fig. 3), and the independent relationship between sclera permeability coefficients and permeant lipophilicities (Fig. 4). The binding of lipophilic permeants to the sclera could also account for the higher effective porosities of sclera calculated by the permeants than that by water, irrespective of their molecular sizes and charges. This is the first systematic study of permeants with a large range of lipophilicities using human sclera, and the permeant uptake results are consistent with the findings in a previous study using bovine sclera (9).
In addition to permeant lipophilicity, permeant-to-sclera charge-charge interactions can also play a role in permeant uptake into and transport across the sclera. At pH 7.4 in the present study, the negatively charged human sclera (15) is permselective to cations. As a result, the charge-charge interaction between the sclera and positively charged permeant could enhance permeant partitioning into the sclera. The higher partition coefficient of positively charged atenolol than negatively charged salicylate is consistent with the charge-charge interaction. This charge-charge interaction effect also accounts for the higher partition coefficient of metoprolol than fluconazole in the present study. The effect of the charge-charge interaction was not observed in the present transport study possibly due to the larger inherited experimental variability in the transport experiments than that in the uptake experiments.
Theoretically, the binding of a permeant to a membrane increases the transport lag time of the permeant (13) as illustrated by the binding factor R in Eq. 10. The results in the present uptake and transport studies are consistent with the hypothesis that higher permeant lipophilicity could lead to larger permeant binding to the sclera (i.e., higher partition coefficient due to the binding), and therefore longer transport lag time. Moreover, the slopes of the linear regression lines in Figs. 1 and 3 are essentially the same, suggesting that the uptake and transport domains (or “binding” sites) in the sclera probed in the uptake and transport experiments are similar. It should be emphasized that the steady-state permeability would not be affected by the binding of permeant to sclera (Eq. 9), and therefore not sensitive to the permeant lipophilicity. Fig. 4 shows no correlation between the permeability coefficient and Doct, which is consistent with the theory (13).
Implications of Permeant Lipophilicity in Transscleral Transport Studies
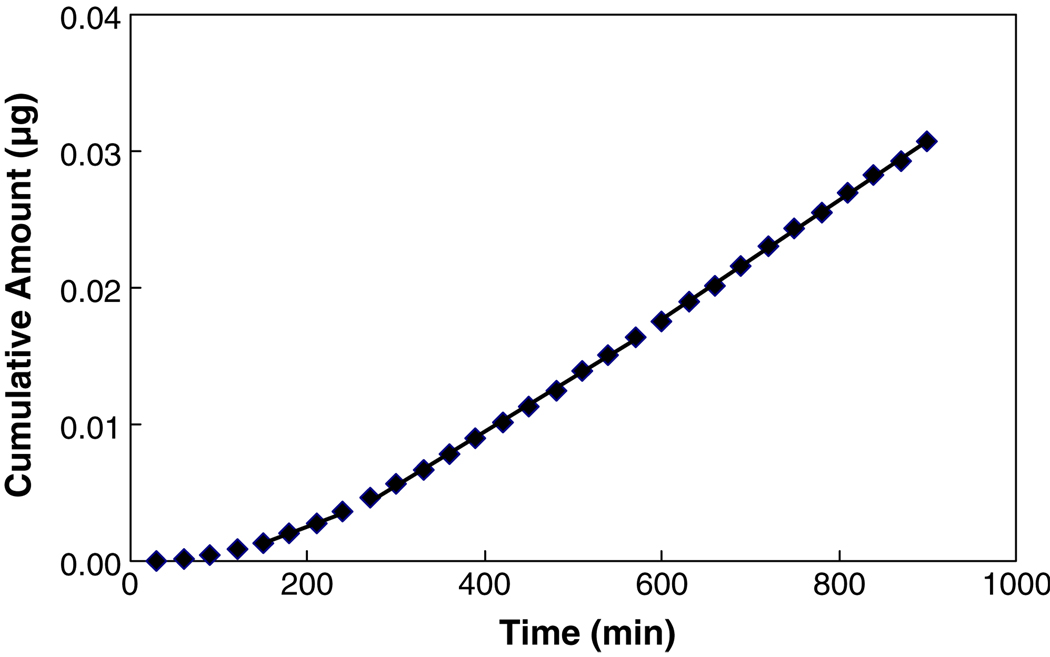
Transport experiments using diffusion cells are commonly employed to evaluate the permeability of the sclera in transscleral drug delivery in vitro. The steady-state permeability coefficient and lag time are usually calculated from the permeation profile of the drug and equations similar to Eqs. 3 and 4. However, a common problem encountered in these transport experiments is that lipophilic permeants usually have long transport lag times, and steady-state transport may not be attained in the experiments. To illustrate this, the lag times and permeability coefficients calculated from three different time regions in each transport experiment for the permeants are presented and compared in Table III. The results in the table show that for hydrophilic permeants like urea, the lag time and permeability are essentially the same in the three time regions (p>0.05); steady state could be reached quickly due to the lack of permeant-to-tissue binding. On the other hand, for permeants with high lipophilicity, such as estradiol, the steady state occurs only after the binding or the interaction between the permeant and sclera has attained “equilibrium” during the transport process, and the lag time and permeability coefficient calculated in Region III (steady-state region) are significantly higher than those in Region I. In the case of estradiol, the lag time and permeability in Region III were about 3.2 and 1.5 times larger than those in Region I, respectively. The most drastic effect of permeant binding on the transport behavior of lipophilic permeants can be illustrated by the transport profile of cyclosporin A in Fig. 5. At around 150–240 min into the experiment, a linear region seemed to be observed in the cumulative amount versus time curve. However, as the time of the experiment prolonged, the curve would continue to ascend, and the “steady state” seemed to be achieved only after about 600 min. The lag times and permeability coefficients calculated from these three regions yield significantly different values.
Table III.
Comparison of Transport Lag Times (min) and Permeability Coefficients (× 10−6 cm/sec) of Urea, Atenolol, Corticosterone, Estradiol, and Cyclosporin A Obtained from Three Time Regions in Their Permeation Profiles
| Region I | Region II | Region III | |
|---|---|---|---|
| Urea | 15–45 min | 45–75 min | 75–120 min |
| Lag Time | 5 ± 2 | 5 ± 1 | 6 ± 2 |
| Permeability Coefficient | 74 ± 20 | 76 ± 20 | 76 ± 20 |
| Atenolol | 15–45 min | 45–75 min | 75–150 min |
| Lag Time | 10 ± 2 | 17 ± 8 | 17 ± 4 |
| Permeability Coefficient | 25 ± 8 | 25 ± 8 | 29 ± 8 |
| Corticosterone | 30–90 min | 90–150 min | 150–360 min |
| Lag Time | 16 ± 5 | 22 ± 8 | 24 ± 11 |
| Permeability Coefficient | 29 ± 11 | 31 ± 10 | 31 ± 9 |
| Estradiol | 30–90 min | 90–150 min | 150–360 min |
| Lag Time | 16 ± 2 | 29 ± 6 | 52 ± 10 |
| Permeability Coefficient | 32 ± 6 | 38 ± 4 | 47 ± 10 |
| Cyclosporin A | 150–240 min | 270–570 min | 600–900 min |
| Lag Time | 93 ± 9 | 161 ± 28 | 189 ± 54 |
| Permeability Coefficient | 2.0 ± 0.9 | 3.0 ± 0.9 | 3.5 ± 0.8 |
Data represent the mean and standard deviation of four sclera samples for each permeant
Fig. 5.
A representative plot of cumulative amount of cyclosporin A transported across human sclera versus time. The lines are the linear regression lines of the data in three time regions of the permeation profile, demonstrating that transport experiments of different durations would lead to different linear regression slopes calculated from the data in these experiments.
The analyses of Table III and Fig. 5 demonstrate that the lag times and permeability coefficients calculated could be highly dependent on the duration of the transport study and the lipophilicity of the permeant, and the lag time and steady-state permeability coefficient could be underestimated when the duration of the experiment is not long enough to reach steady state. Although long duration transport studies are needed to ensure steady-state transport for the accurate determination of the steady-state permeability coefficient and lag time for the understanding of transscleral transport mechanism and the influence of permeant lipophilicity, it should be pointed out that the results from short duration experiments could be used to predict drug delivery in practice. In other words, the apparent fluxes and permeability coefficients obtained in short duration experiments can resemble the practical situation better.
Implications of Drug Lipophilicity in Transscleral Drug Delivery
The retention time of drugs on the eye after topical application is generally short (a few minutes) (16,17). The present study shows that the lag time for drugs with high lipophilicity is likely to be significantly longer than the contact time. Consequently, steady-state delivery of lipophilic drugs is difficult to achieve in practical applications, and the observed fluxes of these lipophilic drugs would be significantly lower than those predicted from their steady-state values. This also implies that in transscleral drug delivery of lipophilic drugs, the amounts of drugs permeated across human sclera are small, and this can limit the efficacy of drug treatment. As a result, sustained-release transscleral delivery systems are required for drugs with high lipophilicity to prolong the retention time, provide extended release, and therefore, enhance drug delivery.
Based on the relationship between drug lipophilicity and partitioning to sclera observed in the present study, drug reservoirs could form in the sclera in transscleral delivery of lipophilic drugs. The lipophilic drugs would accumulate in the sclera (i.e., high effective porosity in the sclera, Table II) and provide sustained delivery from the scleral depots. Transscleral delivery of lipophilic drugs can therefore be improved by formulations to take advantage of this effect. For example, a lipophilic drug with low water solubility could be formulated in an ocular delivery system such as micelles. The system can be delivered transsclerally into the porous sclera. The drug released from the system could then bind to the sclera. A drug depot is formed in the sclera, and the drug is released from the depot in an extended manner. It should be emphasized that although the sclera can serve as a reservoir for lipophilic drugs for transscleral delivery, the retinal pigment epithelium (RPE) and dynamic barriers such as lymph and choroidal blood flow may prevent effective drug delivery using this method. The present in vitro experimental setup with human sclera does not allow the study of these barriers. A model mimicking practical situations is required to further investigate the transscleral route for effective drug delivery to the retina.
Several of the permeants studied in the present paper were shown to be effective drugs for treating eye diseases, and therefore, the present uptake and transport data can serve as the database for transscleral delivery of these drugs. For example, β-blockers including atenolol and metoprolol are widely used for eye diseases like glaucoma (18). Corticosteroids like dexamethasone are effective in treating diabetic macular edema (19). Cyclosporin A has been studied for treating ocular inflammatory diseases (20,21). Fluconazole is an antifungal agent demonstrating effects on endogenous fungal endophthalmitis (22,23). Paclitaxel has the potential use in drug-eluting glaucoma drainage device (24). The findings in the partitioning and transport behaviors of these drugs would be beneficial in clinical applications. Understanding the influences of drug lipophilicity on drug partitioning and drug transport across human sclera would help scientists in designing drugs of desirable properties for transscleral delivery to treat eye diseases.
CONCLUSION
The partitioning of permeants of different lipophilicities into human sclera and their transscleral transport behaviors were studied. The results indicate that permeants with higher lipophilicities exhibit higher partition coefficients, stronger binding to human sclera, and longer transport lag times than polar permeants in transscleral delivery. The results also suggest that steady-state transport might not be easily attained for highly lipophilic permeants in the diffusion cell experiments with human sclera. For human sclera at pH 7.4, the partitioning of positively charged permeants into the sclera was higher than those of neutral and negatively charged permeants, possibly due to charge-charge interactions. The steady-state permeability coefficients of permeants were not significantly affected by the permeant lipophilicity in the present transscleral transport study.
ACKNOWLEDGEMENTS
This research was supported by NIH grant EY 015181. The authors acknowledge the use of tissues procured by the National Disease Research Interchange (NDRI) with support from NIH grant 5 U42 RR006042. The authors also thank Dr. Paul Bernstein and Moran Eye Center at the University of Utah for generously supplying us with some of the sclera tissues used in this study and Poonam Chopra for providing the transscleral transport data of tetraethylammonium, salicylate, and mannitol.
REFERENCES
- 1.Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev. 2001;52:37–48. doi: 10.1016/s0169-409x(01)00193-4. [DOI] [PubMed] [Google Scholar]
- 2.Eljarrat-Binstock E, Pe’er J, Domb AJ. New techniques for drug delivery to the posterior eye segment. Pharm Res. 2010;27:530–543. doi: 10.1007/s11095-009-0042-9. [DOI] [PubMed] [Google Scholar]
- 3.Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 4.Cheruvu NPS, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipophilicity and the choroid-Bruch’s layer. Invest Ophthalmol Vis Sci. 2006;47:4513–4522. doi: 10.1167/iovs.06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–2079. doi: 10.1016/j.addr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci. 2006;47:4532–4539. doi: 10.1167/iovs.06-0030. [DOI] [PubMed] [Google Scholar]
- 8.Cruysberg LP, Nuijts RM, Gilbert JA, Geroski DH, Hendrikse F, Edelhauser HF. In vitro sustained human transscleral drug delivery of fluorescein-labeled dexamethasone and methotrexate with fibrin sealant. Curr Eye Res. 2005;30:653–660. doi: 10.1080/02713680590968600. [DOI] [PubMed] [Google Scholar]
- 9.Kadam RS, Kompella UB. Influence of lipophilicity on drug partitioning into sclera, choroid-retinal pigment epithelium, retina, trabecular meshwork, and optic nerve. J Pharmacol Exp Ther. 2010;332:1107–1120. doi: 10.1124/jpet.109.161570. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 11.Foster CS, de la Maza MS. Structural considerations of the sclera, in: The Sclera. New York: Springer-Verlag; 1994. pp. 1–32. [Google Scholar]
- 12.Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388:107–113. doi: 10.1016/j.ijpharm.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn GL, Yalkowsky SH, Roseman TJ. Mass transport phenomena and models: theoretical concepts. J Pharm Sci. 1974;63:479–510. doi: 10.1002/jps.2600630403. [DOI] [PubMed] [Google Scholar]
- 14.Chopra P, Hao J, Li SK. The 2008 AAPS Annual Meeting and Exposition. Atlanta, Georgia: 2008. Effects of electroosmosis and electrophoresis in iontophoretic transport across human sclera. [Google Scholar]
- 15.Li SK, Zhang Y, Zhu H, Higuchi WI, White HS. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J Pharm Sci. 2005;94:847–860. doi: 10.1002/jps.20293. [DOI] [PubMed] [Google Scholar]
- 16.Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems-recent advances. Prog Retin Eye Res. 1998;17:33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 17.Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–515. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 18.Cheong HI, Johnson J, Cormier M, Hosseini K. In vitro cytotoxicity of eight beta-blockers in human corneal epithelial and retinal pigment epithelial cell lines: comparison with epidermal keratinocytes and dermal fibroblasts. Toxicol In Vitro. 2008;22:1070–1076. doi: 10.1016/j.tiv.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 20.Shii D, Nakagawa S, Shinomiya K, Yoshimi M, Katsuta O, Oda T, et al. Cyclosporin A eye drops inhibit fibrosis and inflammatory cell infiltration in murine type I allergic conjunctivitis without affecting the early-phase reaction. Curr Eye Res. 2009;34:426–437. doi: 10.1080/02713680902866980. [DOI] [PubMed] [Google Scholar]
- 21.Kacmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas PA. Fungal infections of the cornea. Eye (London) 2003;17:852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 23.Habib FS, Fouad EA, Abdel-Rhaman MS, Fathalla D. Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta Ophthalmol. doi: 10.1111/j.1755-3768.2009.01584.x. in press. [DOI] [PubMed] [Google Scholar]
- 24.Choritz L, Grub J, Wegner M, Pfeiffer N, Thieme H. Paclitaxel inhibits growth, migration and collagen production of human Tenon’s fibroblasts-potential use in drug-eluting glaucoma drainage devices. Graefes Arch Clin Exp Ophthalmol. 2010;248:197–206. doi: 10.1007/s00417-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright SH, Wunz TM, Wunz TP. Structure and interaction of inhibitors with the TEA/H+ exchanger of rabbit renal brush border membranes. Pflugers Arch. 1995;429:313–324. doi: 10.1007/BF00374145. [DOI] [PubMed] [Google Scholar]
- 26.Lombardo F, Shalaeva MY, Tupper KA, Gao F. ElogD(oct): a tool for lipophilicity determination in drug discovery. 2. Basic and neutral compounds. J Med Chem. 2001;44:2490–2497. doi: 10.1021/jm0100990. [DOI] [PubMed] [Google Scholar]
- 27.Frum Y, Bonner MC, Eccleston GM, Meidan VM. The influence of drug partition coefficient on follicular penetration: in vitro human skin studies. Eur J Pharm Sci. 2007;30:280–287. doi: 10.1016/j.ejps.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Lucangioli SE, Kenndler E, Carlucci A, Tripodi VP, Scioscia SL, Carducci CN. Relation between retention factors of immunosuppressive drugs in microemulsion electrokinetic chromatography with biosurfactants and octanol-water partition coefficients. J Pharm Biomed Anal. 2003;33:871–878. doi: 10.1016/s0731-7085(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 29.Moosavi-Movahedi AA, Hakimelahi S, Chamani J, Khodarahmi GA, Hassanzadeh F, Luo FT, et al. Design, synthesis, and anticancer activity of phosphonic acid diphosphate derivative of adeninecontaining butenolide and its water-soluble derivatives of paclitaxel with high antitumor activity. Bioorg Med Chem. 2003;11:4303–4313. doi: 10.1016/s0968-0896(03)00524-8. [DOI] [PubMed] [Google Scholar]
- 30.Geiser L, Henchoz Y, Galland A, Carrupt PA, Veuthey JL. Determination of pKa values by capillary zone electrophoresis with a dynamic coating procedure. J Sep Sci. 2005;28:2374–2380. doi: 10.1002/jssc.200500213. [DOI] [PubMed] [Google Scholar]
- 31.Vincent-Ballereau FN, Patey ON, Lafaix C. Fluconazole. Review and situation among antifungal drugs in the treatment of opportunistic mycoses of human immuno-deficiency virus infections. Pharm Weekbl Sci. 1991;13:45–57. doi: 10.1007/BF01974981. [DOI] [PubMed] [Google Scholar]