Abstract
α-crystallins are small heat-shock proteins important to lens transparency that provide the lens with its refractive properties. In their role as molecular chaperones, these crystallins also prevent protein aggregation, affect cytoskeletal remodeling, enhance resistance to cell stress, and provide lens cells with protection against apoptosis. While many of the functions assigned to αA-crystallin are attributable to its presence in the cytoplasm of lens cells, αA-crystallin also has been detected at the lens plasma membrane. However, how αA-crystallin becomes linked to the plasma membrane or what its functions are at this site has remained unknown. In this study, we examined the mechanisms by which αA-crystallin becomes associated with the lens membrane, focusing specifically on its interaction with membrane receptors, and the differentiation-specificity of these interactions. We also determined how the long-term absence of αA-crystallin alters receptor-linked signaling pathways. αA-crystallin association with membrane receptors was determined by co-immunoprecipitation analysis; its membrane localization was examined by confocal imaging; and the effect of αA-crystallin loss-of-function on the activation state of signaling molecules in pathways linked to membrane receptors was determined by immunoblot analysis. The results show that, in lens epithelial cells, plasma membrane αA-crystallin was primarily localized to apicolateral borders, reflecting the association of αA-crystallin with E-cadherin complexes. These studies also provide the first evidence that αA-crystallin maintained its association with the plasma membrane in lens cortical fiber cells, where it was localized to lateral interfaces, and further show that this association was mediated, in part, by αA-crystallin interaction with α6 integrin receptor complexes. We report that the absence of αA-crystallin led to constitutive activation of the stress kinases p38 and JNK, classical inducers of apoptotic cell death, and the loss of the phospho-Bad pro-survival signal, effects that were greatest in differentiating lens fiber cells. Concurrent with this, activation of FAK and ERK kinases was increased, demonstrating that these receptor-linked pathways also were dysregulated in the absence of αA-crystallin. These data link αA-crystallin plasma membrane association to its differentiation-state-specific interaction with E-cadherin and α6 integrin receptor complexes. The changes in cell signaling in αA-crystallin-null lenses suggest that dysregulation of receptor-linked cell-signaling pathways that accompany the failure of αA-crystallin to associate with membrane receptors may be responsible for the induction of apoptosis. The observed changes in lens cell signaling likely reflect long-term functional adaptations to the absence of the αA-crystallin chaperone/small heat-shock protein.
Keywords: chaperone, lens, apoptosis, integrin, ERK, p38, JNK
INTRODUCTION
Lens α-crystallin consists of two proteins, αA-crystallin and αB-crystallin, each encoded by a different gene. These proteins, originally identified for their role in lens transparency, contribute to lens refractive properties. Loss of αA-crystallin, such as in the lenses of αA-crystallin knockout (αA−/ −) mice, causes αB-crystallin to accumulate as aggregates, leading to the light scattering associated with formation of cataracts (Brady et al., 1997). Since the original discovery of these crystallins in the lens, αB-crystallin has been found to be present in many other cells and tissues (Bhat and Nagineni, 1989; Dubin et al., 1989), whereas αA-crystallin has a more limited distribution (Sax and Piatigorsky, 1994). In a seminal study, Horwitz showed that the α-crystallins have important functions beyond the structural role that provides for transparency, showing that these crystallins were, in fact, small heat-shock proteins that functioned as molecular chaperones (Horwitz, 1992). In this capacity, the α-crystallins have been shown to prevent protein aggregation (Horwitz, 2000); confer cells with the ability to resist cell stress (Andley et al., 1998; Andley et al., 2000); and remodel/protect microfilament, microtubule and intermediate filament cytoskeletons (Nicholl and Quinlan, 1994; Andley et al., 1998; Muchowski et al., 1999; Head et al., 2000; Xi et al., 2006).
Chaperone proteins prevent non-specific protein aggregation by maintaining their substrate proteins in a folded conformation, and by directing misfolded proteins to the proteasome for degradation (Boelens et al., 2001; Nollen and Morimoto, 2002; Den Engelsman et al., 2003; Arrigo, 2007). When chaperone activity is compromised, misfolded or damaged proteins accumulate, potentially stimulating stress-activated MAPK (mitogen-activated protein kinase) cascades, such as the p38 and c-Jun N-terminal kinase (JNK) pathways, each of which can impact the cellular decision to survive or die (Nollen and Morimoto, 2002; Launay et al., 2006; Arya et al., 2007). The ability to provide cells with a mechanism to resist apoptosis is among the most important chaperone functions of α-crystallins (Andley et al., 1998; Andley et al., 2000; Xi et al., 2003). This protective role of α-crystallins has been demonstrated in lenses, where loss of αA- and/or αB-crystallins, or the presence of a mutant αA-crystallin, enhances the susceptibility of epithelial and fiber cells to apoptotic cell death (Andley et al., 2000; Andley et al., 2001; Xi et al., 2003; Morozov and Wawrousek, 2006; Xi et al., 2008). These finding are also strongly supported by studies on lens cells cultured in vitro (Andley et al., 2000; Andley et al., 2001; Andley et al., 2002; Morozov and Wawrousek, 2006). Furthermore, the ability of αA-crystallin to enhance cell survival in response to stress has been linked to its interaction with the pro-apoptotic molecules Bax and Bcl-Xs, and activation of an Akt survival signal, whereas αB-crystallin is linked to cell survival through an ERK signaling pathway (Liu et al., 2004). Thus, the loss of αA-crystallin may cause the loss of survival signals as well as stimulate stress signaling. Although the link between α-crystallins and resistance to cell death is strong, the mechanism by which αA-crystallin regulates cell survival signaling pathways has remained obscure.
Molecular chaperones such as αA-crystallin have co-evolved with signaling proteins to coordinate the responses of cells to environmental cues (Gaestel, 2002; Nollen and Morimoto, 2002). Such cellular responses to the environment typically involve the engagement and activation of plasma membrane-linked receptors. But to date, there have been no reports of α-crystallin association with such receptors, and α-crystallin function has been attributed mostly to its actions in the cytoplasm. Significant interactions of αA-crystallin with lens membranes have been reported to occur with cataract onset, but this association results from changes in the biochemical properties of lens membranes that are acquired with aging (Ifeanyi and Takemoto, 1990b; Ifeanyi and Takemoto, 1990a; Ifeanyi and Takemoto, 1991; Cobb and Petrash, 2000; Cobb and Petrash, 2002). While αA-crystallin also has been shown to localize along cell-cell borders of normal lens epithelial cells (Wang et al., 2004), how αA-crystallin is targeted to the lens plasma membrane is not yet known, and no studies have investigated whether αA-crystallin associates with membrane receptors that regulate cell survival signaling pathways.
One potential membrane receptor to which αA-crystallin might be targeted is α6 integrin, a receptor that confers survival properties on cells (Friedland et al., 2007). Many integrins are expressed by lens cells, but only α6 integrin has been linked to lens cell differentiation (Walker et al., 2002a). This integrin localizes to the basal membranes of lens cells, where it interacts with laminins in the surrounding lens basement membrane capsule, and along the cell-cell interfaces of lens epithelial and fiber cells (Walker et al., 2002c). Both α6A and α6B isoforms of this integrin subunit are expressed in the lens, but in a differentiation-state-specific manner, switching from α6B to α6A as lens cell differentiation is initiated (Walker and Menko, 1999). This pattern of α6 integrin isoform-switching parallels that which occurs in the differentiation of embryonic stem cells (Cooper et al., 1991). Recruitment of the insulin-like growth factor 1 receptor (IGF-1R) to α6 integrin receptor complexes at the onset of lens cell differentiation links the lens α6 integrin receptor to classical IGF-1R cell survival signaling pathways (Walker et al., 2002c). The predicted role of this α6 integrin/IGF-1R survival signaling complex in the lens is to regulate the induction of the caspase-3 differentiation-initiation signal by the canonical mitochondrial death pathway, allowing this novel signaling pathway to act as a molecular switch in the initiation of lens cell differentiation without causing apoptosis (Weber and Menko, 2005).
E-cadherin, which has been linked to cell survival signaling through phosphoinositide 3-kinase (Calautti et al., 2005), is another membrane receptor that is a potential binding partner for αA-crystallin at the plasma membrane. E-cadherin is a member of a receptor family that has been implicated in guiding the morphogenesis of epithelial tissues (Gumbiner, 1996; Gumbiner, 2005). In the lens, the dramatic changes in cellular architecture (Piatigorsky, 1981) that accompany lens cell differentiation and development have been linked to cadherin function (Calautti et al., 2005; Leonard et al., 2008). Lens cells express multiple cadherins, but the best studied are E-cadherin, which is found primarily in lens epithelial cells (Xu et al., 2002; Pontoriero et al., 2009), and N-cadherin, expressed both by lens epithelial and fiber cells (Leong et al., 2000; Beebe et al., 2001; Xu et al., 2002; Pontoriero et al., 2009). The absence of both E-cadherin and N-cadherin in double E-cadherin/N-cadherin conditional knockout lenses causes defects in cell adhesion and survival of lens epithelial cells (Pontoriero et al., 2009). A role for cadherins in lens cell differentiation and morphogenesis has been shown only for N-cadherin (Ferreira-Cornwell et al., 2000).
Here, we examined the mechanisms underlying αA-crystallin association with the lens membrane, focusing specifically on the membrane receptors that αA-crystallin interacts with and the differentiation-specificity of these interactions. We also determined how the long-term absence of αA-crystallin in the lens of αA−/− mice alters receptor-linked signaling pathways. We found that plasma membrane αA-crystallin in lens epithelial cells is principally localized along the apicolateral borders, reflecting recruitment by E-cadherin complexes. We also provide the first evidence for plasma membrane-localized αA-crystallin in lens cortical fiber cells, showing that αA-crystallin is present at the lateral interfaces of the plasma membrane, where it is maintained, at least in part, through interactions with α6 integrin receptor complexes. The chronic absence of αA-crystallin was associated with constitutive activation of the stress kinases p38 and JNK, classical inducers of apoptotic cell death, and loss of phospho-Bad pro-survival signals. These effects were greatest in differentiating lens fiber cells, linking αA-crystallin association with the plasma membrane to a differentiation state-specific interaction with E-cadherin and α6 integrin receptor complexes. The absence of αA-crystallin was also associated with increased activation of receptor-linked FAK and ERK kinases. The changes in cell signaling observed in αA−/− lenses suggest that the failure of αA-crystallin to associate with membrane receptors and the resulting dysregulation of receptor-linked cell-signaling pathways may be responsible for the induction of apoptosis. The observed changes in lens cell signaling likely reflect long-term functional adaptations to the absence of the αA-crystallin chaperone/small heat-shock protein.
MATERIALS AND METHODS
Animals and tissue dissection
129SvEv (wild-type) mice from Taconic Farms (Hudson, NY, USA) and αA-crystallin knockout (αA−/−) mice, provided by Eric Wawrousek (National Eye Institute, Bethesda, MD, USA), were used in this study. All animal protocols were in accordance with the Washington University institutional policy on the use of animals in research. Inbred mice were used at 7–10 weeks of age. After euthanizing mice by CO2 inhalation, whole eyes were removed, and whole lenses dissected into epithelial and cortical fiber cell populations, as shown in Figure 2C. In experiments using embryonic day 10 chicken lenses, lenses were microdissected prior to analysis into four distinct regions of differentiation: central epithelial cells, equatorial epithelial cells, cortical fiber cells, and nuclear fiber cells (Figure 3B).
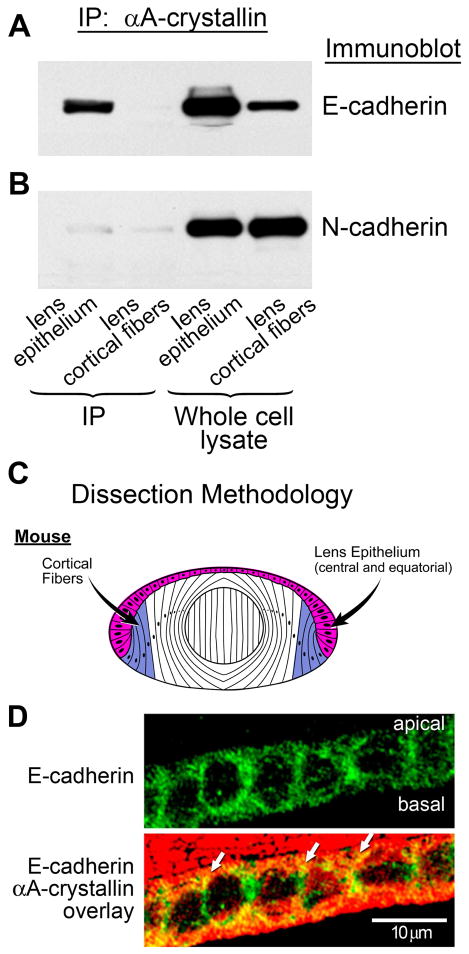
Figure 2.
Association of αA-crystallin with E-cadherin in lens epithelial cells. (A) Co-immunoprecipitation analysis of lens extracts following immunoprecipitation with an antibody to αA-crystallin, and immunoblotting with an antibody to E-cadherin. Note the strong association of αA-crystallin with E-cadherin in the lens epithelium, but no detectable association in lens cortical fibers. (B) Co-immunoprecipitation analysis of lens extracts following immunoprecipitation with an antibody to αA-crystallin and immunoblotting with an antibody to N-cadherin. Note that the association of N-cadherin with αA-crystallin was very low in both lens epithelium and lens cortical fiber cells. Whole cell lysates demonstrate the relative levels of E- and N-cadherin expression in lens epithelium and cortical fibers. (C) Diagram of dissection scheme for obtaining lens epithelial (red) and cortical fiber (blue) cells for these analyses from mouse lenses. In these preparations, the lens epithelial fraction comprised both central and equatorial epithelial cells. (D) Transverse sections of cortical lens fiber cells in adult wild-type mouse lenses were double stained for E-cadherin and αA-crystallin. E-cadherin was detected all along the lateral interfaces of these lens epithelial cells; αA-crystallin co-localized with E-cadherin (white arrows) particularly at the apical borders of these cells.
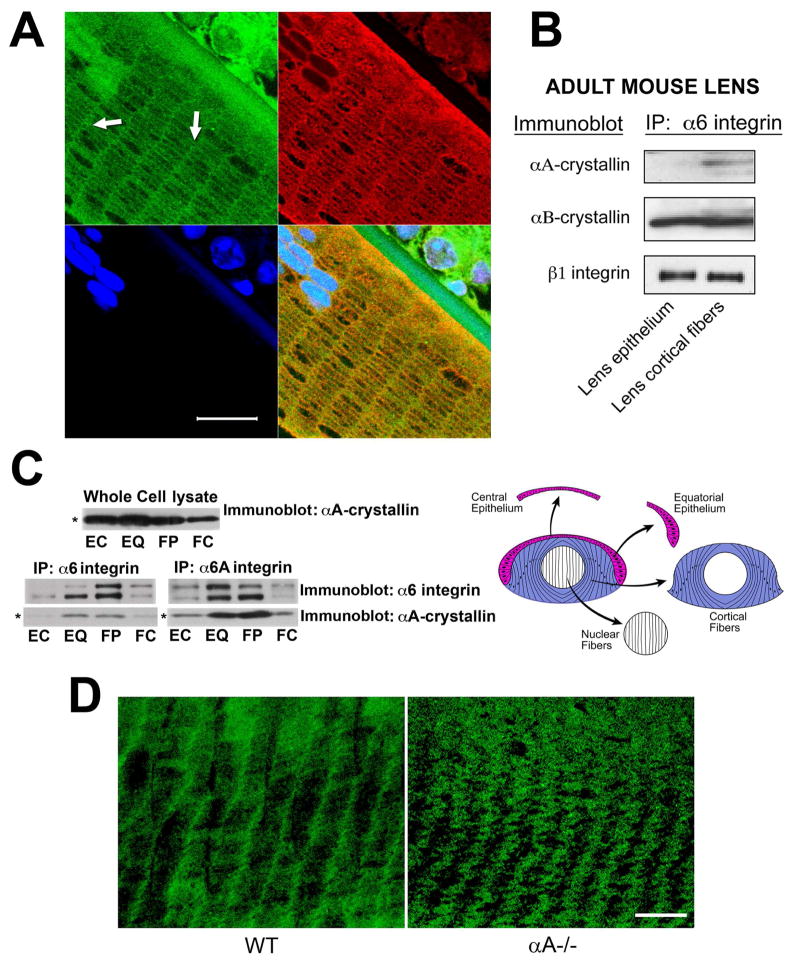
Figure 3.
Differentiation-state-specific association of αA-crystallin with α6 integrin complexes. (A) Confocal microscopic analysis of cross-sections of cortical lens fiber cells double labeled with antibodies to α6 integrin and αA-crystallin. α6 integrin localized all along the cell-cell interfaces of these hexagonally-packed cortical fiber cells, but was predominantly associated with the short arms of the hexagon. αA-crystallin was co-localized with α6 integrin along these lateral interfaces (white arrows). The green staining of nuclei at the top left of the image is due to non-specific staining with the α6 integrin antibody. DNA was stained with draq5(blue). Scale bar = 20 μm. (B) Adult mouse lenses were dissected into epithelial and cortical fiber fractions as diagrammed in Figure 2C. Cell extracts were immunoprecipitated with an antibody to α6 integrin, and immunoblotted with antibodies to αA-crystallin, αB-crystallin, and ß1 integrin. Note that αA-crystallin association with α6 integrin was specific to cortical fiber cells, while the association of αB-crystallin with α6 integrin occurred in both lens epithelial and cortical fiber cells. (C) Chicken embryo lenses were dissected into four differentiation state-specific fractions: central epithelium (EC, red), equatorial epithelium (EQ, red), cortical fiber cells (FP, blue), and nuclear fiber cells (FC, white), as diagrammed in C. Cell extracts were immunoprecipitated either with an antibody to α6 integrin (recognizes both α6A and α6B integrin isoforms) or an antibody specific to α6A integrin, and immunoblotted with antibodies to αA-crystallin and α6 integrin. Note that the association of αA-crystallin with α6 integrin in these embryonic lenses was high in the zones of lens cell differentiation, including cells in the equatorial epithelium (EQ) and cortical fiber zones (FP). Note that the whole cell lysate (upper panel), had nearly equal levels of αA-crystallin in the central epithelium, equatorial epithelium and the cortical fiber cells. (D) Confocal microscopic analyses were performed on cortical fiber cells adjacent to the epithelium in wild-type (WT) and αA−/− lenses following immunostaining for α6 integrin. Note that α6 integrin was localized primarily to the short arms of these hexagonally packed fiber cells in wild-type lenses. While α6 integrin remained associated with the short arms of cortical fiber cells in αA−/− lenses, its linear organization at the plasma membrane was lost and these integrin junctions exhibited a wavy, disorganized pattern at the plasma membrane. Scale bar = 20 μm.
Immunofluorescence studies
Mouse eyes were fixed, embedded in paraffin and 3-μm sections were prepared for examination of lens fiber cells as either transverse or cross-sections. For examination of lens epithelium, tissue from this region of the lens was isolated, fixed, and observed as whole mounts as described previously (Xi et al., 2003). Paraffin sections were deparaffinized and hydrated in deionized water, and treated with Tris-Glycine buffer, pH 8.0 for 2 hours. After washing with water, they were pretreated with citrate buffer, pH 6.0 and antigen retrieval was performed in a Biocore medical decloaking chamber. Sections were washed and incubated in 20% inactivated donkey serum. Tissue sections were immunostained using a monoclonal antibody to αA-crystallin (1:200 dilution) provided by Dr. Paul FitzGerald (University of California, Davis, CA, USA), rat monoclonal antibody (1:200 dilution) to α6 integrin (GoH3; BD-Pharmingen, San Jose, CA, USA), antibody to E-cadherin (Cell Signaling Technology, Danvers, MA, USA), and antibody to αA/αB-crystallin (Andley et al., 1996) overnight at 4°C, and washed four times for 15 minutes with PBS. After incubating with primary antibody, tissue sections were incubated in 20% normal goat serum for 20 min and treated with a secondary Alexa568-conjugated goat anti-mouse IgG (1:300 dilution), for one hour at room temperature (Andley et al., 1998), or Alexa488-conjugated goat anti-rat IgG (1:500 dilution), as appropriate. Nuclei were stained with TOTO-1 (Molecular Probes, Eugene, OR, USA) or DRAQ5 (Cell Signaling). Sections were washed four times for 15 minutes with PBS, and cover slips were attached (Vectashield hardset (Vector Laboratories, Burlingame, CA, USA). Immunostained sections were visualized using a Zeiss LSM 510 confocal microscope (Zeiss, Jena, Germany) using a 63x objective. Primary quail lens epithelial cell cultures were immunostained with antibodies to α6 integrin and αA-crystallin by methods described previously (Walker et al., 2002b)
Immunoblot analysis
Freshly dissected mouse lens epithelial or cortical fiber cell tissue, pooled from at least 12 mouse lenses, was analyzed by immunoblotting. Samples were extracted in lysis buffer (44.4 mM n-octyl β-D-glucopyranoside, 1% Triton X-100, 100 mM NaCl, 1 mM MgCl2, 5 mM EDTA, 10 mM imidazole) containing 1 mM sodium vanadate, 0.2 mM H2O2, and protease inhibitor cocktail (Sigma, St. Louis, MO, USA), as previously described (Weber and Menko, 2005). Equal amounts of total cellular protein (15 μg) were separated on Tris-glycine gels (Novex, San Diego, CA, USA), electrophoretically transferred to a membrane (Immobilon-P, Millipore Corp., Bedford, MA, USA), and immunoblotted as described previously (Weber and Menko, 2005). Primary antibodies against the following proteins/protein forms were used for immunoblot analyses: Ser112-phosphorylated Bad (pBadSer112; Biosource, Camarillo, CA, USA); p38 (Santa Cruz Biotechnology); phosphorylated p38 (p-p38), phosphorylated ERK1/2 (p-ERK1/2) and ERK1/2 (Promega, Madison, WI, USA); JNK (Millipore) and phosphorylated JNK (p-JNK; Invitrogen); FAK (Santa Cruz Biotechnology); and phosphorylated FAK397 (p-FAK397; BD BioScience). All gels were run under reducing conditions. Densitometric analyses were performed using Kodak 1D software (Eastman Kodak Company, Rochester, NY, USA).
Immunoprecipitation analysis
A co-immunoprecipitation assay was used to investigate the association of αA-crystallin with α6 integrin, E-cadherin, and N-cadherin receptor complexes in epithelial and cortical fiber fractions of wild-type mouse lenses (diagrammed in Figure 2C) or microdissected fractions from chick embryo lenses representing four distinct regions of differentiation (diagrammed in Figure 3B). Lens fractions were extracted in lysis buffer (described above), and extracts containing 100 βg protein were incubated with primary antibody. Immunoprecipitates were collected with protein G resin (Sigma) and resolved by SDS-PAGE, as described previously (Walker et al., 2002a; Walker et al., 2002c). Whole-cell lysates (10 βg protein) were run in parallel. Immunoprecipitations from chick lens fractions were performed as previously described (Walker et al., 2002a; Walker et al., 2002c). Antibody-alone controls were included for all immunoprecipitation studies, and in all cases the controls were negative (data not shown). After separating on SDS-polyacrylamide gels, proteins were electrophoretically transferred onto Immobilon-P membranes (Millipore), and co-immunoprecipitation was determined by immunoblot analysis, as described previously (Walker et al., 2002a; Walker et al., 2002c). Antibodies against the following proteins were used in these studies: E-cadherin, N-cadherin, ß1 integrin (BD-Transduction, San Jose, CA), α6 integrin (BD-Pharmingen, San Jose, CA, USA), α6 integrin (Developmental Studies Hybridoma Bank), α6A integrin (Santa Cruz Biotechnology), αA-crystallin (generously provided by Dr. Paul FitzGerald, University of California, San Francisco, CA, USA), and αB-crystallin (Stressgen Biotechnologies, Victoria, Canada).
Primary quail embryo lens cultures
Primary cultures of differentiating quail lens cells were prepared as described previously (Weber and Menko, 2005). Briefly, lens cells were isolated from quail lenses at embryonic day 10 by trypsinization and agitation. Isolated cells were plated on laminin and cultured in Medium 199 with 10% fetal bovine serum. For studies with phorbol 12-myristate 13-acetate (PMA), primary quail embryo lens cell cultures were exposed to 15 nM PMA in normal culture media for 4-5 hours, as indicated. Control cultures were exposed to the vehicle dimethylsulfoxide (DMSO).
Cell transfection
cDNA encoding human wild-type αA-crystallin was cloned into the pCIneo mammalian expression vector (Promega) and transfected into human lens epithelial cells (HLE B-3), as described previously (Andley et al., 1998; Andley et al., 2002). The R116C mutation was introduced using the QuikChange Site-Directed Mutagenesis Kit, according to the manufacturer's recommendations (Stratagene, Agilent Technologies, Wilmington, DE, USA). The sequence of each construct was confirmed by sequencing on an ABI 310 genetic analyzer using a dRhodamine Dideoxy-terminator Cycle Sequencing kit (Perkin-Elmer Life Sciences, Waltham, MA, USA). Human lens epithelial cells with extended life span (HLE B-3) were cultured in 20% fetal bovine serum containing Eagle's minimum essential medium and gentamicin (50 μg/ml), as described previously (Andley et al., 1994). These cells were passaged through at least 11 passages, after which they ceased to produce αA-crystallin, as determined by immunoblot analysis (Fleming et al., 1998). Thus, introduction of wild-type or R116C αA-crystallin cDNA into these cells after passage 11 by cDNA transfection followed by growth in selection medium can be used to generate stable cell lines expressing different levels of αA-crystallin. Transfections were carried out using the Promega ProFection kit according to the manufacturer's recommendations, and cells were treated with a DMSO shock treatment after 16 hours of transfection to increase transfection efficiency (Andley et al., 2002). After 48 hours, cells were trypsinized and subcultured (1:3), and Geneticin was added at a concentration of 0.5 mg/ml for colony selection. Geneticin-resistant colonies were isolated, and individual colonies were expanded into mass cultures. Each colony, presumably derived from a single cell, was examined by quantitative immunoblot analysis and compared with cells transfected with vector only. The expression of αA-crystallin was unchanged over four passages, as determined by immunoblot analysis. Cell lines expressing wild-type αA-crystallin or the R116C mutant at 2.5 ng/μg cellular protein were used. Clonal cell lines were used for two passages for immunoblot analysis of signaling proteins.
RESULTS
Localization of αA-crystallin to cell-cell interfaces in both lens epithelial and fiber cells
We investigated the association of αA-crystallin with the plasma membrane of the normal lens by confocal microscopy imaging, and determined whether the membrane localization of αA-crystallin was differentiation-state specific. To best image the different regions of the lens the epithelium was examined as tissue whole mounts, and the cortical fiber cells in tissue sections cut both in both transverse and cross-sectional planes. Images of undifferentiated lens epithelial cells were acquired at focal planes located near both the cells’ apical and basal domains. αA-crystallin staining was intense along the apicolateral cell-cell borders of the lens epithelium (Figure 1A), in a polarized pattern similar to that reported for junctional proteins in these cells (Leonard et al., 2008; Pontoriero et al., 2009). In the basal half of the cells (i.e., in the same focal plane as the cell nuclei), αA-crystallin was principally cytoplasmic (Figure 1B), as has been reported previously (Xi et al., 2003). Imaging of αA-crystallin in tissue cross-sections of cortical fiber cells provided the first evidence that this crystallin was also localized to lateral cell-cell interfaces in differentiating lens fiber cells (Figure 1C). Immunostaining of lateral sections through the cortical fiber region demonstrated that αA-crystallin was localized to cell-cell interfaces all along the lateral borders of these differentiating lens fiber cells (Figure 1D). The membrane localization of αA-crystallin in cortical fiber cells is similar to that reported for N-cadherin and for membrane proteins of the integrin receptor family (Walker and Menko, 1999; Leonard et al., 2008).
Figure 1.
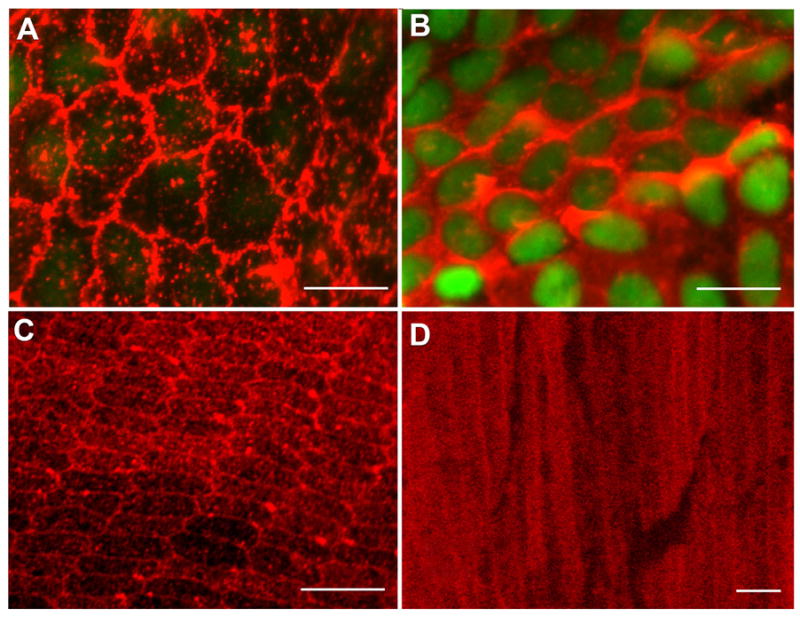
Confocal micrographs of αA-crystallin expression in the mouse lens. (A, B) Lens epithelial whole mounts prepared from adult wild-type mouse lenses were fixed and then incubated with a monoclonal antibody specific to αA-crystallin, followed by incubation with an Alexa568-conjugated secondary antibody (red); nuclei were stained with TOTO-1 (green). Cells were imaged by confocal microscopy at both apical (A) and basal (B) domains. Cells in the central epithelium are shown. (A) Note the expression of αA-crystallin on regions of plasma membrane at apical cell-cell interfaces. (B) Nuclei (green) are localized to the basal aspects of the lens epithelium where αA-crystallin was primarily cytoplasmic. Adult mouse lenses, ~150-day-old, were used to prepare lens epithelial whole mounts. (C) Cross-sectional and transverse sections of cortical lens fiber cells in adult wild-type mouse lenses. Cross-sectional slices were cut in the lens equatorial plane. Sections were stained with a monoclonal antibody to αA-crystallin and an Alexa568-conjugated secondary antibody (red). Intense staining for αA-crystallin was observed at the fiber cell interfaces in the outer cortical, hexagonally packed fiber cells. The periphery of the lens is at the bottom of the image. (D) In transverse sections the staining at cell-cell interfaces was shown to extend all along the lateral cell-cell borders of the cortical fiber cells. Cytoplasmic staining also was seen, which increased in the central fiber cells. Distance from the center of the lens is 400 μm. Scale bars = 10 μm (A), 20 μm (B, C), and 5μm (D).
Association of αA-crystallin with E-cadherin receptor complexes in the lens epithelium
We next investigated whether αA-crystallin recruitment to the plasma membrane of lens epithelial and/or fiber cells reflected its association with cadherin receptor complexes. For these studies, mouse lens epithelial and cortical fiber cell zones were first isolated by microdissection, and then cadherin expression was examined by immunoblotting for E- and N-cadherins. E-cadherin, the classical cadherin of epithelial cells, was expressed principally by cells of the lens epithelium and at lower levels in cortical fiber cells (Figure 2A), whereas N-cadherin was highly expressed in both lens epithelial and cortical fiber cells (Figure 2B), as shown previously (Leong et al., 2000; Xu et al., 2002; Pontoriero et al., 2009). Co-immunoprecipitation analysis (immunoprecipitate: αA-crystallin; immunoblot: cadherin) revealed that the localization of αA-crystallin to the plasma membrane of lens epithelial cells reflected its recruitment to E-cadherin junctions (Figure 2A). Despite the high level of expression of N-cadherin in the lens epithelium, only a low level of association between αA-crystallin and N-cadherin was detected. In the cortical lens fiber cell zone, there was little association detected between αA-crystallin and N-cadherin (Figure 2B) or E-cadherin (Figure 2A). The lack of association between N-cadherin and αA-crystallin in lens epithelial cells is particularly striking when compared to the high degree of αA-crystallin interaction with E-cadherin junctions in lens epithelial cells, and provides evidence of the specificity of αA-crystallin association with E-cadherin in lens epithelial cells. Although E-cadherin can be detected all along the lateral interfaces of the mouse lens epithelial cells, the co-localization of E-cadherin with αA-crystallin was greatest at the apicolateral borders of these cells (Figure 2D).
Differentiation-state-specific association of αA-crystallin with α6 integrin
A strong candidate for the recruitment of αA-crystallin to the fiber cell plasma membrane (Figure 1C, D) was α6 integrin, a receptor subunit required for lens cell differentiation (Walker et al., 2002a; Walker et al., 2002c). α6 integrin localizes to the cell-cell interfaces of differentiating lens fiber cells (Cooper et al., 1991; Walker and Menko, 1999), a region that is devoid of matrix, along with β1 integrin (Menko and Philip, 1995; Walker and Menko, 1999), an integrin subunit with which α6 integrin forms a heterodimeric receptor (Cooper et al., 1991; Walker and Menko, 1999). To investigate more closely where α6 integrin was localized along the lateral cell borders of the hexagonally packed fiber cells we performed confocal microscopic analysis on cross-sections of cortical lens fiber cells immunostained with antibody to α6 integrin. This study provided the first evidence that α6 integrin was localized all along the cell-cell interfaces of these hexagonally-shaped cortical fiber cells, but was predominantly associated with the short arms of the hexagon (Figure 3A). This localization pattern was similar to that observed for other adhesion complex molecules (Straub et al., 2003). Co-immunostaining of lens cross-sections with antibody to αA-crystallin and α6 integrin demonstrated that αA-crystallin co-localized with α6 integrin along the lateral cell-cell borders of cortical lens fiber cells (Figure 3A).
To investigate whether αA-crystallin was specifically recruited to the plasma membrane of differentiating lens fiber cells through its association with α6 integrin, co-immunoprecipitation analyses were performed on epithelial and cortical fiber cell fractions isolated from mouse lenses. While no interaction between αA-crystallin and α6 integrin was detected in mouse lens epithelial cells, αA-crystallin was linked to α6 integrin receptor complexes in cortical fiber cells (Figure 3B), consistent with a role in lens fiber cell differentiation. Different results were obtained for αB-crystallin, which co-precipitated with α6 integrin in both undifferentiated lens epithelial and differentiating lens fiber cells. The efficiency of α6 integrin immunoprecipitation in both lens epithelial and cortical fiber cells was demonstrated by immunoblotting for its heterodimeric partner ß1 integrin (Figure 3B).
A similar association of αA-crystallin with α6 integrin receptor complexes in differentiating fiber cells was observed in chick embryo lenses (Figure 3C). For these studies, embryonic day 10 chicken lenses were microdissected into four distinct regions of differentiation, making it possible to further specify the differentiation zone in which the association of αA-crystallin with the plasma membrane could be attributed to its linkage to α6 integrin receptor complexes. As shown in Figure 3C, the four regions yielded by microdissection include: undifferentiated cells of the anterior epithelium; the equatorial epithelium (the region of differentiation initiation); cortical fiber cells (the principal region of lens fiber cell differentiation); and nuclear lens fiber cells (the most differentiated cells of the embryonic lens). These co-immunoprecipitation studies were performed with two different α6 integrin antibodies, one that recognizes both the α6A and α6B integrin isoforms, the other an antibody that is specific to the differentiation-specific α6A integrin isoform. The results were similar with both antibodies, revealing that as differentiation is initiated in the region of the equatorial epithelium the association of αA-crystallin with the plasma membrane can be attributed to its linkage to α6 integrin receptor signaling complexes and that this linkage remained high in the cortical fiber zone of lens fiber cell differentiation (Figure 3C).
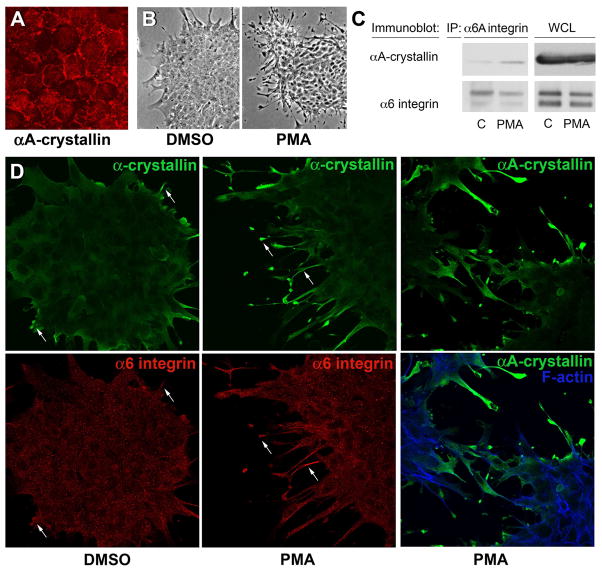
To investigate the factors that determine the recruitment of αA-crystallin to α6 integrin receptor complexes, we examined whether association between these proteins was induced following α6 integrin activation. For these studies, we took advantage of the ability of PMA to activate α6 integrin receptors through an inside-out signaling event that involves the signaling intermediate protein kinase C (Shaw and Mercurio, 1993). Primary quail lens epithelial cell cultures were used for these studies, which shared many properties with lens cells in vivo including localization of αA-crystallin to peripheral membranes (Figure 4A). The quail lens epithelial cells were cultured in the presence of PMA, or its vehicle, DMSO. For these studies the cells were cultured on a substrate composed of the basal lamina protein laminin, the principal α6 integrin extracellular matrix ligand. The efficacy of α6 integrin activation by PMA was determined by evaluating the ability of the treated cells to extend lamellipodial/filopodial processes along this laminin substrate. Exposure of lens epithelial cells to PMA induced extensive elaboration of lamellipodial/filopodial filaments along the laminin substrate (Figure 4B). While exposure of the lens primary cultures to PMA induced activation of α6 integrin, it did not alter α6 integrin expression, as evidenced by direct immunoblot analysis of whole-cell lysates (Figure 4C). The effect of activation of α6 integrin by PMA on the recruitment of αA-crystallin to α6 integrin signaling complexes was determined by co-immunoprecipitation analyses. For these studies α6 integrin receptor complexes were isolated by immunoprecipitation with antibodies to the α6A integrin subunit and then immunoblotted with antibody to αA-crystallin. This co-immunoprecipitation analysis demonstrated that activation of α6A integrin by PMA induced recruitment of αA-crystallin to α6 integrin signaling complexes (Figure 4C).
Figure 4.
PMA activation of α6 integrin induces its association with αA-crystallin. (A) Confocal imaging of quail embryo lens epithelial cell cultures immunostained for αA-crystallin (red) showed αA-crystallin localized to cell-cell interfaces, demonstrating that these cells are a good model for studying the mechanism of αA-crystallin association with the plasma membrane. The dependence of αA-crystallin recruitment to α6 integrin on the activation state of this integrin was examined using PMA to activate α6 integrin. Control cultures were treated with DMSO. (B) Efficacy of PMA activation of the α6 integrin receptor was demonstrated by the elaboration of extensive lamellipodial/filopodial processes by the lens epithelial cells along a laminin coated substrate. (C) Recruitment of αA-crystallin to activated α6A integrin signaling complexes was determined following immunoprecipitation of α6 integrin with an antibody to the α6A subunit. Immunoprecipitates were immunoblotted with antibodies to both α6 integrin and αA-crystallin. PMA exposure induced αA-crystallin recruitment to α6 integrin complexes. (D) Co-localization of a-crystallins and α6 integrin in lamellipodial/filopodial processes extended in the presence of PMA was demonstrated by co-immunostaining. Cultures treated with either DMSO or PMA were double-labeled with antibodies to α6 integrin and α-crystallin (αA/αB-crystallin), or singly labeled with antibody to αA-crystallin and double stained with a nuclear marker. Both α-crystallins and α6 integrin were prominently localized to the processes the lens epithelial cells extended on the laminin substrate (arrows).
The effect of PMA on αA-crystallin association with α6 integrin was examined further by co-immunolocalization analysis. Confocal image analysis was performed on PMA- and DMSO-treated lens cell cultures that were double-labeled with antibodies to α6 integrin and α-crystallin (αA- and αB-) and also on parallel cultures labeled with antibody to αA crystallin (due to properties of the antibodies it was not possible to double label for α6 integrin and αA-crystallin). The results demonstrated the PMA exposure induced co-localization of α-crystallins and α6-integrin in the lamellipodial/filopodial processes that had been elaborated by the lens epithelial cells along the laminin substratum, and the specific localization of αA-crystallin to these cellular extensions (Figure 4D).
These studies demonstrated that αA-crystallin was highly associated with α6 integrin receptor complexes in differentiating lens fiber cells, and showed that this recruitment was dependent on the activation state of α6 integrin. Next, using confocal imaging, we examined the influence of αA-crystallin chaperone function at the plasma membrane of differentiating cortical fiber cells on the membrane distribution and organization of α6 integrin receptors. For this investigation, cross-sections of wild-type and αA−/− mouse lenses were immunostained with an antibody to α6 integrin. Although α6 integrin remained associated with the short arms of cortical fiber cells in the αA−/− lenses, it lost its linear distribution and adopted a disorganized appearance in the membrane (Figure 3D).
Effects of long-term absence of αA-crystallin on signaling pathways associated with cell death and survival in lens epithelial and fiber cells
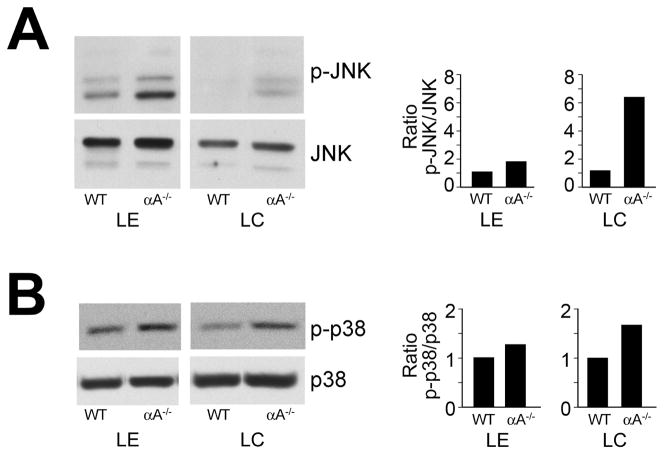
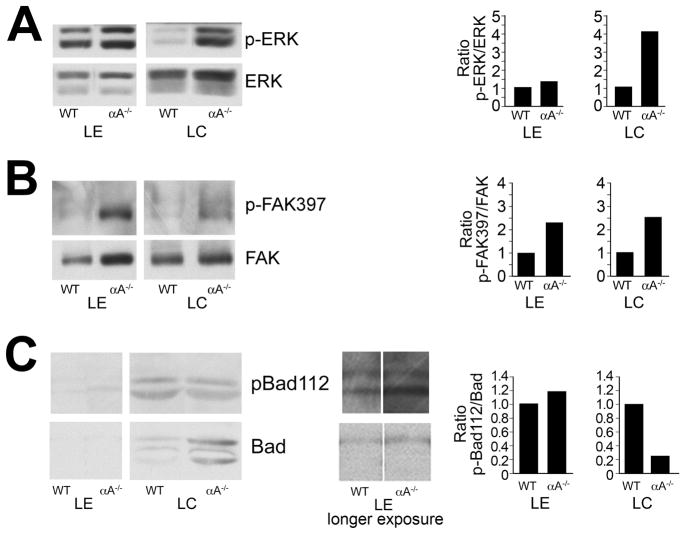
These studies demonstrate for the first time that the association of αA-crystallin with the plasma membrane involves E-cadherin in undifferentiated lens epithelial cells and α6 integrin during lens fiber cell differentiation. Both these receptors are central regulators of cell signaling and, like αA-crystallin, have been shown to provide cells with resistance to apoptosis. These properties led us to investigate whether the long-term absence of αA-crystallin disrupted or activated signaling pathways associated with cell survival or cell death. Western blot analyses using phospho-specific antibodies that recognize the active state of the stress-activated MAPKs p38 and JNK were performed to examine whether the absence of αA-crystallin chaperone function in the lens induced activation of stress signaling pathways. The relative activation state of p38 and JNK in isolated lens epithelial and cortical fiber zones from 2-month-old αA−/− mice was compared to that in similar fractions from wild-type age-matched lenses. For these studies, the lens differentiation-stage-specific zones represent fractions isolated from multiple lenses to control for signaling variations between animals. Therefore, the data represents consistent trends in the changes in activation state of signaling molecules that were observed in the absence of αA-crystallin. In lenses from αA−/− mice, p38 and JNK were constitutively activated in both lens epithelial and cortical fiber cells (Figure 5), whereas total levels of p38 and JNK protein were unchanged. The most significant effects on stress kinase activation occurred in the cortical fiber cells.
Figure 5.
Activation of JNK and p38 stress pathways in αA−/− lens epithelial and fiber cells. Lens epithelial (LE) and cortical fiber cell (LC) fractions were dissected from 18–20 wild-type (WT) or αA−/− mouse lenses. Cell extracts were examined by immunoblot analysis with antibodies to (A) p-JNK and JNK, and (B) p-p38 and p38. Note that while p38 and JNK were activated in lens epithelial and cortical fiber cells of αA−/− mice, the degree of activation of these stress kinases was much greater in the cortical fiber zone of αA−/− lenses. Protein bands were quantified, and ratios of activated to total protein were determined and presented as fold-change between αA−/− and WT lenses. The results are representative of three independent experiments.
Focal adhesion kinase (FAK) and the MAP kinase ERK are classical cell survival signaling molecules. An alternative and contradictory role for ERK in the lens also has been reported – that of mediator of apoptosis through upregulation of p53 (Liu et al., 2004; Ahmed et al., 2005). ERK and FAK were activated in the lens in response to the loss of αA-crystallin in both lens epithelial and fiber cells, although for ERK the greatest response was in the cortical fiber cells (Figure 6A and 6B).
Figure 6.
Changes in survival signaling in αA−/− lens epithelial and fiber cells. Lens epithelial (LE) and cortical fiber cell (LC) fractions were dissected from 18–20 wild-type or αA−/− mouse lenses. Cell extracts were examined by immunoblot analysis with antibodies to (A) p-ERK and ERK1/2, (B) p-FAK397 and FAK, and (C) pBad112 and Bad. The results show increased activation of both ERK and FAK in αA−/− lens epithelial and cortical fiber cells, but decreased phosphorylation of Bad in the cortical fiber zone. Protein bands were quantified, and the ratios of activated to total protein were determined and represented as fold-change between αA−/− and WT lenses. The results are representative of three independent experiments.
Because phosphorylation of Bad is necessary for this Bcl-2 family member to function as a pro-survival signal (Chipuk et al., 2004), we compared the phosphorylation state of Bad (pBad112/total Bad) in αA−/− lenses to its phosphorylation state in wild-type control lenses to evaluate the effect of αA-crystallin ablation on lens-survival signaling. This analysis was performed for both the lens epithelial and cortical fiber zones. The absence of αA-crystallin in the lens epithelium had little effect on Bad phosphorylation, but did cause a decrease in Bad phosphorylation in lens cortical fiber cells (Figure 6C). The decreased level of Bad phosphorylation was due in part to increased expression of Bad, likely a cellular response to the loss of phosphorylated Bad.
Activation of the p38 stress kinase by the human cataract-causing αA-R116C mutant
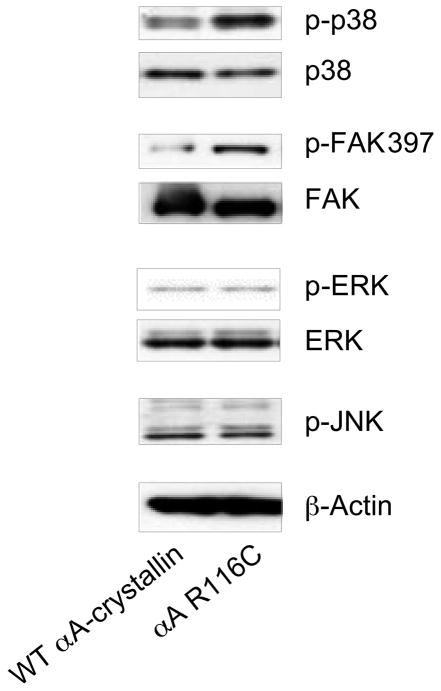
To further examine the link between the loss of αA-crystallin function and activation of stress pathways in the lens, we compared the activation state of the stress kinases p38 and JNK in human lens epithelial (HLE) cells expressing the αA-crystallin R116C mutant with that in HLE cells expressing equivalent levels of wild-type αA-crystallin ((see Materials and Methods and (Andley et al., 2002) for analysis of expression levels)). Immunoblot analyses using phospho-specific antibodies to p38 and JNK showed that the presence of the αA-crystallin R116C mutant induced activation of the stress kinase p38, but had little effect on the activation state of JNK (Figure 7). The αA-crystallin R116C mutant had no detectable effect on the activation of ERK, but did induce activation of FAK. These results show that changes in αA-crystallin function caused by mutation enhanced both cell survival and apoptosis-promoting pathways in lens epithelial cells, but in a more selective manner than that caused by αA-crystallin deletion (αA−/− lenses).
Figure 7.
The αA-crystallin R116C mutant induces activation of p38 and FAK. Extracts of HLE B-3 lens epithelial cells transfected with either wild-type αA-crystallin or the αA-crystallin R116C mutant were examined by immunoblot analysis with antibodies to p-p38, p38, pFAK397, FAK, p-ERK, ERK, p-JNK, and β-actin. Expression of the αA-crystallin R116C mutant in lens epithelial cells induced significant activation of FAK and the stress kinase p38 compared with lens epithelial cells transfected with wild-type αA-crystallin. There was little difference in the activation state of JNK or ERK between αA-crystallin R116C- and WT-transfected lens epithelial cells.
DISCUSSION
We report here that deletion of the αA-crystallin gene causes activation of stress-signaling pathways in the lens. In cortical lens fiber cells, this activation of p38 and JNK stress kinases correlated with the abnormal plasma membrane distribution of α6 integrin. This result was particularly interesting in light of our discovery that, in addition to its well-established cytoplasmic distribution, αA-crystallin was localized to the plasma membrane along the cell-cell interfaces of cortical fiber cells in wild-type lenses. This plasma membrane localization of αA-crystallin involved recruitment to α6 integrin receptor complexes. The localization of αA-crystallin to the cell-cell borders of the differentiating cortical fiber cells, is similar to that previously reported for both α6 and β1 integrins in newly forming lens fiber cells (Menko and Philip, 1995; Walker and Menko, 1999), and we now show that αA-crystallin co-localizes with α6 integrin along the lateral borders of these hexagonally packed lens fiber cells. This association of αA-crystallin with α6 integrin receptor complexes in lens cells could be induced by activating α6 integrin with PMA, supporting the conclusion that αA-crystallin has a role in regulating α6 integrin-induced signaling pathways in the lens.
The α-crystallins have been identified as survival-promoting molecules that protect cells from apoptosis (Andley, 2007); αA-crystallin serves this function in the lens (Andley et al., 1998; Xi et al., 2003), and αB-crystallin acts in this capacity in numerous cell types, including the lens (Andley et al., 2000; Kamradt et al., 2001; Arrigo et al., 2002; Stegh et al., 2008; Shin et al., 2009). Similarly, α6 integrin-mediated signaling complexes activate survival signaling pathways that provide resistance to apoptosis (Colognato et al., 2002; Friedland et al., 2007). In the developing lens, this involves α6 integrin linkage to the IGF-1 receptor, a classical survival molecule (Walker et al., 2002c). Our studies show that both αA- and αB-crystallins were associated with α6 integrin in cortical lens fiber cells. The link between α6 integrin and survival signaling in the lens suggests that the association of α-crystallins with α6 integrin in the cortical lens fiber zone reflects their role in protecting lens fiber cells from apoptotic death in response to stress. However, our discovery that αA-crystallin first became associated with α6 integrin signaling complexes as lens cells initiated their differentiation program indicates that the chaperone function of αA-crystallin also may play an important role in the survival signaling pathways that have been implicated in regulating the lens differentiation-inducing caspase-3 signal. Particularly interesting in the context of our results is the observation that cell death induced in the lens fiber region is linked to the activation of caspase-3 in lenses of αA/αB double-knockout mice (Morozov and Wawrousek, 2006). In addition, αB-crystallin inhibits apoptosis in other cell types in response to stress by preventing the autoproteolytic maturation of caspase-3 (Kamradt et al., 2001; Stegh et al., 2008; Shin et al., 2009). This property of α-crystallins provides further support for our hypothesis that the association of αA-crystallin with α6 integrin in differentiating cortical fiber cells of the lens reflects αA-crystallin function in the α6 integrin-linked survival-signaling pathway that regulates the caspase-3 differentiation-initiation signal.
In undifferentiated lens epithelial cells αA-crystallin also was localized to cell-cell interfaces, but its distribution was polarized, principally apicolateral, and this localization reflected its recruitment to E-cadherin receptor complexes. Interestingly, while αA-crystallin was highly linked to E-cadherin complexes there was little association of αA-crystallin with N-cadherin, another major cadherin expressed by lens epithelial cells. The similarity between the cytoplasmic domains of E- and N-cadherins suggests that the selective recruitment of αA-crystallin crystallin to E-cadherin junctional complexes involves an indirect linkage, such as through a specific cadherin complex protein like the catenins. Relevent to this point, αB-crystallin contains ß-catenin-interacting sequences (Ghosh et al., 2007).
The results of this study indicate that the protection of lens cells by αA-crystallin involves suppression of p38 and JNK stress kinase signaling pathways. The activation of the p38 and JNK stress pathways was greatest in αA−/− lens fiber cells and occurred in association with diminished phosphorylation of the Bcl-2 family protein Bad at serine 112, indicating concurrent loss of a key cell survival signal. These results suggest that the αA-crystallin chaperone function has a particularly important, and possibly singular, role in protecting lens fiber cells against the activation of stress pathways that induce cell death. This conclusion is supported by previous studies that indicate a role for αA-crystallin in protecting fiber cells from apoptosis. These studies have demonstrated substantial disorganization and morphological aberrations in the fiber cells of αA−/− lenses (Brady et al., 1997), and shown significant levels of cell death in the fiber cells of lenses from αA/αB-crystallin double-knockout mice (Morozov and Wawrousek, 2006). More direct evidence for a protective function of αA-crystallin as a survival molecule in lens fiber cells comes from knock-in studies with the R49C αA-crystallin mutant and show that αA-crystallin protects lens fiber cells as well as lens epithelial cells from apoptotic cell death (Brady et al., 1997; Arrigo et al., 2007; Xi et al., 2008). Our finding that the loss of αA-crystallin had a greater effect on stress kinase activation in lens fiber cells than in lens epithelial cells is likely attributable to the presence of redundant survival pathways in lens epithelial cells. These redundancies in survival signaling reflect the fact that the lens epithelium is the first line of defense in the lens against environmental stresses, such as UV exposure. As such, this important cell layer must be able to respond adroitly to the multiple stress factors that challenge the lens. Cell death in the lens epithelium can cause the formation of opacities associated with formation of cortical cataracts (Li et al., 1995; Zhou et al., 2007).
The sustained loss of αA-crystallin in αA−/− mice also led to constitutive activation of the ERK MAP kinase. While ERK activation is most commonly identified as a survival signal, in cultured lens cells, it has been reported that the induction of apoptosis by UVA stress is associated with activation of ERK, downstream of Raf (Liu et al., 2004). αB-crystallin also has been shown to attenuate apoptosis by inhibiting an ERK-activated p53 pathway (Li et al., 2005). The lens cells examined in the current study are more representative of the in vivo state, which might suggest a survival function for ERK activation in the absence of αA-crystallin. However, because ERK activation associated with the loss of αA-crystallin may be a compensatory response that serves to override the cell-death signals induced by activation of stress kinases, we cannot yet discriminate between possible pro- or anti-apoptotic functions of the ERK activation observed in αA−/− lenses.
The results of the current studies add to our understanding of how αA-crystallin regulates lens cell survival and differentiation pathways. αA-crystallin is an ATP-independent molecular chaperone/small heat-shock protein (Horwitz, 2000). Small heat-shock proteins are physiological regulators that have co-evolved as integral components of signal-transduction pathways (Gaestel, 2002; Nollen and Morimoto, 2002). Consistent with a role for heat-shock proteins and molecular chaperones in signal transduction, Hsp90 and Hsp70 have been shown to associate with a number of signaling molecules (Nollen and Morimoto, 2002). Moreover, decreasing Hsp90 levels causes developmental abnormalities, whereas increasing Hsp70 levels has growth-inhibitory effects. In addition, Hsp27 associates with cytochrome c to prevent activation of procaspase-9, and thereby inhibits cell death (Garrido et al., 1999). Likewise, αB-crystallin blocks caspase-3 activation and prevents apoptosis (Mao et al., 2001). Changes in the relative abundance of chaperones can result in subsets of signaling pathways becoming enhanced, suppressed, or constitutively activated (Mosser and Morimoto, 2004). The absence of αA-crystallin in lens cells due to gene deletion or mutation may increase the levels of destabilized and unfolded proteins, which could cause activation of ERK, JNK, and p38 MAP kinases by destabilizing integrin signaling complexes. Alternatively, the activation of these MAP kinases in these cells may be the result of direct effects of αA-crystallin loss on integrin signaling pathways. Other chaperones are known to be associated with adhesion complexes. αB-crystallin localizes to the lamellipodia of lens epithelial cells in an integrin-dependent manner (Maddala and Rao, 2005), and Hsp90 binds to the integrin signaling effector, integrin-linked kinase (Aoyagi et al., 2005). The interactions between αA-crystallin and both E-cadherin and α6 integrin reported here support a direct role for αA-crystallin in the regulation of receptor-linked signaling pathways. More specifically, the cell death observed in the lens in the absence of α-crystallins (Andley et al., 1998; Xi et al., 2003; Morozov and Wawrousek, 2006) and in αA-crystallin mutant transfectants (Andley et al., 2002; Mackay et al., 2003) suggests that αA-crystallin enhances or supports integrin- and cadherin-mediated survival signaling in the lens. Our finding that αA-crystallin was localized to cell-cell borders where it was associated with E-cadherin and α6 integrin molecules in lens epithelial and cortical fiber cells, respectively, integrates these findings into a model of αA-crystallin regulation of survival signals in differentiating lens cells.
In overexpression studies, αA-crystallin prevents stress-induced apoptosis (Andley et al., 1998; Andley et al., 2000). The altered αA-crystallin function of the cataract-causing R116C mutant (Litt et al., 1998) also results in apoptosis in transfected lens epithelial cells (Andley et al., 2002). Our current finding that p38 was activated in cells expressing the αA-crystallin R116C mutant (Figure 7) shows that mutation of αA-crystallin, like deletion of αA-crystallin (Figure 5), leads to misactivation of stress signaling pathways in the lens. In addition, a comparison of the αA−/− lenses with αA-R49C homozygous lenses suggests that the mutation has a stronger effect on fiber cell signaling than simple gene deletion (Brady et al., 1997; Xi et al., 2008). Similarly, loss of αA-crystallin by gene deletion (Brady et al., 1997) does not produce as dramatic an effect on lens morphology as homozygous αA-R49C mutation (Xi et al., 2008). These observations supports the concept that the R49C mutation has a knock-in effect on signaling, producing a gain-of-toxic-function phenotype.
In summary, this study adds to our knowledge of αA-crystallin as a stress sensor and protector of lens cell viability in vivo. This is the first report demonstrating αA-crystallin association with α6 integrin receptors in lens fiber cells in vivo, and linking αA-crystallin with cadherin complexes in lens epithelial cells. We propose that αA-crystallin interacts with the α6 integrin receptor complex in lens fiber cells and functions as a stabilizing molecule for α6 integrin signaling complexes in vivo. Because α6 integrin function is required for lens cell differentiation (Walker and Menko, 1999; Walker et al., 2002c), αA-crystallin may be part of a novel signaling pathway that is required for the maintenance of lens cell survival during differentiation. The changes in signaling pathways in αA-crystallin knockout lenses reported here may reflect long term adaptations to the absence of chaperone activity in lens cells.
Acknowledgments
This work was supported in part by United States National Institutes of Health Grants EY10577 and EY014258 (to A. S. M.), EY05681 (to U.P.A.), and Core Grant EY02687 to the Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, and Research to Prevent Blindness, Inc. We gratefully acknowledge Liping Zhang for her excellent technical assistance, Dr. Janice Walker for insightful discussions regarding our data, Dr. Eric Wawrousek (National Eye Institute, National Institutes of Health, Bethesda, MD, USA) for providing the αA-crystallin knockout mice, and Dr. Paul FitzGerald (University of California, Davis, CA, USA) for providing a monoclonal antibody to αA-crystallin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, Forsberg J, Bergsten P. Protein profiling of human pancreatic islets by two-dimensional gel electrophoresis and mass spectrometry. J Proteome Res. 2005;4:931–940. doi: 10.1021/pr050024a. [DOI] [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphαA-crystallin. J Biol Chem. 1996;271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- Andley UP, Patel HC, Xi JH. The R116C mutation in alpha A-crystallin diminishes its protective ability against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. doi: 10.1074/jbc.M109211200. [DOI] [PubMed] [Google Scholar]
- Andley UP, Rhim JS, Chylack LT, Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphαA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from alphaB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. FASEB J. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Fujita N, Tsuruo T. Stabilization of integrin-linked kinase by binding to Hsp90. Biochem Biophys Res Commun. 2005;331:1061–1068. doi: 10.1016/j.bbrc.2005.03.225. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. The cellular "networking" of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Paul C, Ducasse C, Manero F, Kretz-Remy C, Virot S, Javouhey E, Mounier N, Diaz-Latoud C. Small stress proteins: novel negative modulators of apoptosis induced independently of reactive oxygen species. Prog Mol Subcell Biol. 2002;28:185–204. doi: 10.1007/978-3-642-56348-5_10. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–734. [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Boelens WC, Croes Y, De Jong WW. Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim Biophys Acta. 2001;1544:311–319. doi: 10.1016/s0167-4838(00)00243-0. [DOI] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Petrash JM. Characterization of alpha-crystallin-plasma membrane binding. J Biol Chem. 2000;275:6664–6672. doi: 10.1074/jbc.275.9.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BA, Petrash JM. alpha-Crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry (Mosc) 2002;41:483–490. doi: 10.1021/bi0112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, Ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the alpha 6 beta 1 integrin. J Cell Biol. 1991;115:843–850. doi: 10.1083/jcb.115.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Engelsman J, Keijsers V, De Jong WW, Boelens WC. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J Biol Chem. 2003;278:4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, Menko AS. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Exp Cell Res. 2000;256:237–247. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Song Z, Andley UP. Expression of growth control and differentiation genes in human lens epithelial cells with extended life span. Invest Ophthalmol Vis Sci. 1998;39:1387–1398. [PubMed] [Google Scholar]
- Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- Gaestel M. sHsp-phosphorylation: enzymes, signaling pathways and functional implications. Prog Mol Subcell Biol. 2002;28:151–169. doi: 10.1007/978-3-642-56348-5_8. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. Faseb J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry (Mosc) 2007;46:6308–6317. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Head MW, Hurwitz L, Kegel K, Goldman JE. AlphaB-crystallin regulates intermediate filament organization in situ. Neuroreport. 2000;11:361–365. doi: 10.1097/00001756-200002070-00028. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. The function of alpha-crystallin in vision. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- Ifeanyi F, Takemoto L. Alpha crystallin from human cataractous vs. normal lenses: change in binding to lens membrane. Exp Eye Res. 1990a;50:113–116. doi: 10.1016/0014-4835(90)90017-o. [DOI] [PubMed] [Google Scholar]
- Ifeanyi F, Takemoto L. Specificity of alpha crystallin binding to the lens membrane. Curr Eye Res. 1990b;9:259–265. doi: 10.3109/02713689009044521. [DOI] [PubMed] [Google Scholar]
- Ifeanyi F, Takemoto L. Involvement of the N-terminal region in alpha-crystallin-lens membrane recognition. Exp Eye Res. 1991;53:305–308. doi: 10.1016/0014-4835(91)90234-6. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006;312:3570–3584. doi: 10.1016/j.yexcr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev Biol. 2008;319:298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong L, Menko AS, Grunwald GB. Differential expression of N- and B-cadherin during lens development. Invest Ophthalmol Vis Sci. 2000;41:3503–3510. [PubMed] [Google Scholar]
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16:4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, Et Al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M, Kramer P, Lamorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphαA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphαA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- Maddala R, Rao VP. alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp Cell Res. 2005;306:203–215. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Mao YW, Xiang H, Wang J, Korsmeyer S, Reddan J, Li DW. Human bcl-2 gene attenuates the ability of rabbit lens epithelial cells against H2O2-induced apoptosis through down-regulation of the alpha B-crystallin gene. J Biol Chem. 2001;276:43435–43445. doi: 10.1074/jbc.M102195200. [DOI] [PubMed] [Google Scholar]
- Menko AS, Philip NJ. Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res. 1995;218:516–521. doi: 10.1006/excr.1995.1186. [DOI] [PubMed] [Google Scholar]
- Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphαA-/alphaB-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999;40:951–958. [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–417. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax CM, Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Mercurio AM. Regulation of alpha 6 beta 1 integrin laminin receptor function by the cytoplasmic domain of the alpha 6 subunit. J Cell Biol. 1993;123:1017–1025. doi: 10.1083/jcb.123.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Kim SW, Lim CM, Jeong JY, Piao CS, Lee JK. alphaB-crystallin suppresses oxidative stress-induced astrocyte apoptosis by inhibiting caspase-3 activation. Neurosci Res. 2009;64:355–361. doi: 10.1016/j.neures.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, Louis DN, Chin L, Depinho RA. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci. 2003;116:4985–4995. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- Walker JL, Menko AS. alpha6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhang L, Menko AS. A signaling role for the uncleaved form of alpha 6 integrin in differentiating lens fiber cells. Dev Biol. 2002a;251:195–205. doi: 10.1006/dbio.2002.0823. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhang L, Menko AS. Transition between proliferation and differentiation for lens epithelial cells is regulated by Src family kinases. Dev Dyn. 2002b;224:361–372. doi: 10.1002/dvdy.10115. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhang L, Zhou J, Woolkalis MJ, Menko AS. Role for alpha 6 integrin during lens development: Evidence for signaling through IGF-1R and ERK. Dev Dyn. 2002c;223:273–284. doi: 10.1002/dvdy.10050. [DOI] [PubMed] [Google Scholar]
- Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–3619. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem. 2005;280:22135–22145. doi: 10.1074/jbc.M414270200. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, Andley UP. Reduced survival of lens epithelial cells in the alphαA-crystallin-knockout mouse. J Cell Sci. 2003;116:1073–1085. doi: 10.1242/jcs.00325. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, Mcgaha R, Andley UP. Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. FASEB J. 2006;20:846–857. doi: 10.1096/fj.05-5532com. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Zhou J, Leonard M, Van Bockstaele E, Menko AS. Mechanism of Src kinase induction of cortical cataract following exposure to stress: destabilization of cell-cell junctions. Mol Vis. 2007;13:1298–1310. [PubMed] [Google Scholar]