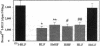Abstract
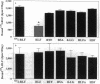
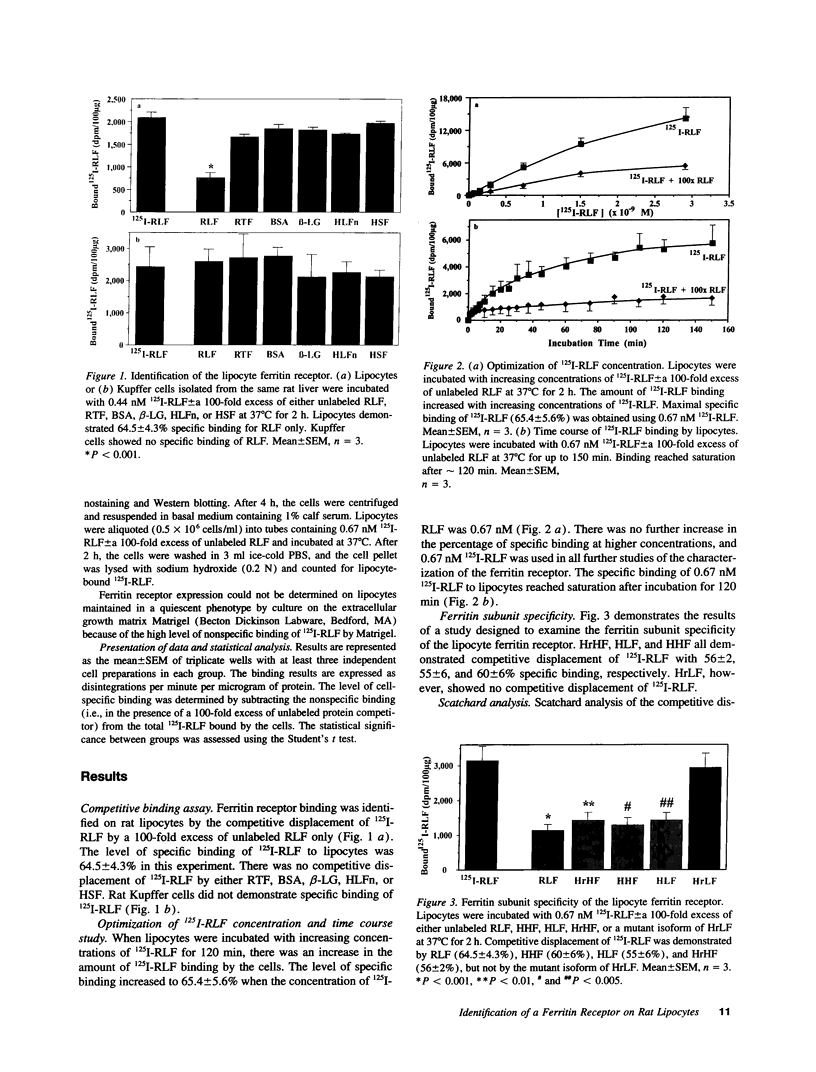
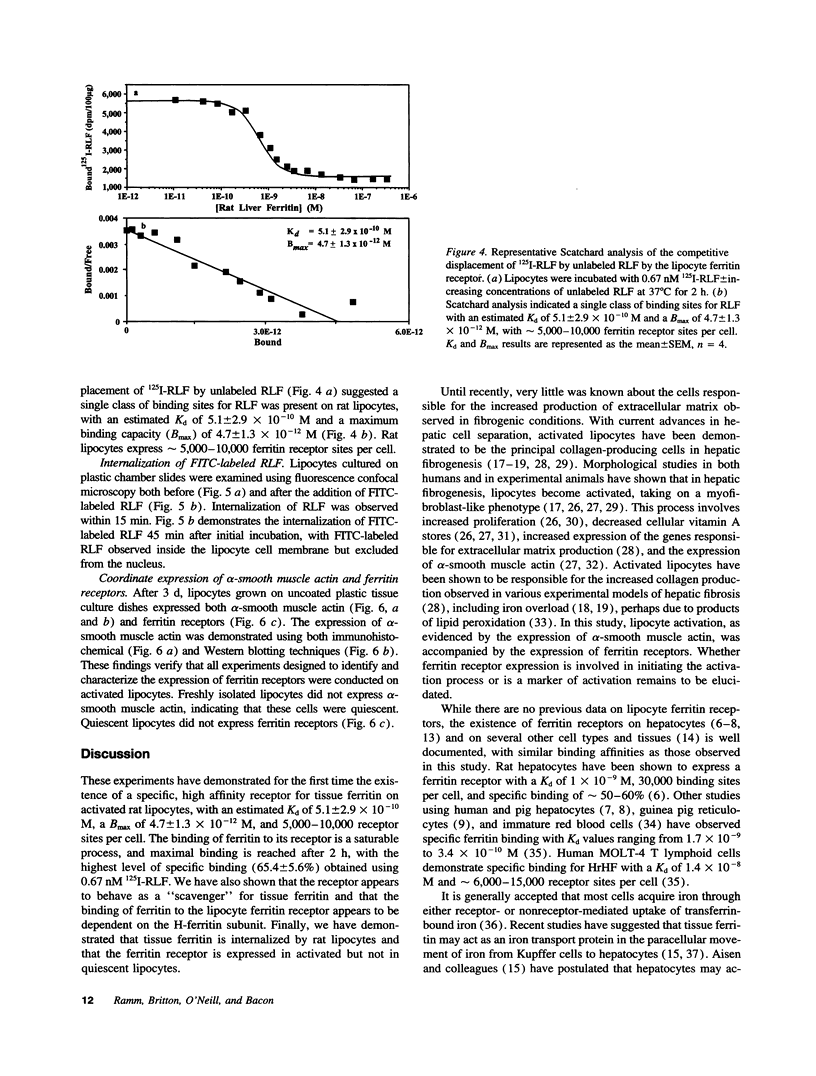
Hepatic iron overload causes lipocyte activation with resultant fibrogenesis. This study examines whether rat lipocytes express ferritin receptors, which could be involved in paracellular iron movement and in cellular regulation. Lipocytes from normal rat liver were cultured on plastic and incubated with 125I-labeled rat liver ferritin (RLF) +/- a 100-fold excess of either unlabeled RLF or human heart ferritin, human liver ferritin, human recombinant H-ferritin, a mutant human recombinant L-ferritin, or a variety of nonspecific proteins. Specific binding sites for ferritin were demonstrated by displacement of 125I-RLF by RLF (64.5 +/- 4.3%) and by other ferritins (55-60%), but not by recombinant L-ferritin. Scatchard analysis demonstrated a single class of binding sites with a Kd of 5.1 +/- 2.9 x 10(-10) M, maximum binding capacity of 4.7 +/- 1.3 x 10(-12) M, and 5,000-10,000 receptor sites/cell. Ferritin receptor expression was observed only in activated lipocytes. Internalization of RLF was observed within 15 min using FITC-RLF and confocal microscopy. This study demonstrates that (a) activated lipocytes express a specific high affinity ferritin receptor; (b) the binding appears to be dependent on the H-ferritin subunit; and (c) lipocytes internalize ferritin. Expression of ferritin receptors in activated lipocytes suggests that the receptor may either be involved in the activation cascade or may be a marker of activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. C., Mack U., Powell L. W., Halliday J. W. Isolation of a porcine hepatic ferritin receptor. Comp Biochem Physiol B. 1988;90(4):837–841. doi: 10.1016/0305-0491(88)90342-2. [DOI] [PubMed] [Google Scholar]
- Adams P. C., Powell L. W., Halliday J. W. Isolation of a human hepatic ferritin receptor. Hepatology. 1988 Jul-Aug;8(4):719–721. doi: 10.1002/hep.1840080402. [DOI] [PubMed] [Google Scholar]
- Anderson G. J., Faulk W. P., Arosio P., Moss D., Powell L. W., Halliday J. W. Identification of H- and L-ferritin subunit binding sites on human T and B lymphoid cells. Br J Haematol. 1989 Oct;73(2):260–264. doi: 10.1111/j.1365-2141.1989.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Blight G. D., Morgan E. H. Receptor-mediated endocytosis of transferrin and ferritin by guinea-pig reticulocytes. Uptake by a common endocytic pathway. Eur J Cell Biol. 1987 Apr;43(2):260–265. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Broxmeyer H. E. H-ferritin: a regulatory cytokine that down-modulates cell proliferation. J Lab Clin Med. 1992 Sep;120(3):367–370. [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Bicknell D. C., Williams D. E., Cooper S., Levi S., Salfeld J., Arosio P. The influence of purified recombinant human heavy-subunit and light-subunit ferritins on colony formation in vitro by granulocyte-macrophage and erythroid progenitor cells. Blood. 1986 Dec;68(6):1257–1263. [PubMed] [Google Scholar]
- Carthew P., Edwards R. E., Smith A. G., Dorman B., Francis J. E. Rapid induction of hepatic fibrosis in the gerbil after the parenteral administration of iron-dextran complex. Hepatology. 1991 Mar;13(3):534–539. [PubMed] [Google Scholar]
- Cazzola M., Bergamaschi G., Dezza L., Arosio P. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: achievements and prospects. Blood. 1990 May 15;75(10):1903–1919. [PubMed] [Google Scholar]
- Chojkier M., Houglum K., Solis-Herruzo J., Brenner D. A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J Biol Chem. 1989 Oct 5;264(28):16957–16962. [PubMed] [Google Scholar]
- Dezza L., Cazzola M., Piacibello W., Arosio P., Levi S., Aglietta M. Effect of acidic and basic isoferritins on in vitro growth of human granulocyte-monocyte progenitors. Blood. 1986 Mar;67(3):789–795. [PubMed] [Google Scholar]
- Fargion S., Cappellini M. D., Fracanzani A. L., De Feo T. M., Levi S., Arosio P., Fiorelli G. Binding and suppressive activity of human recombinant ferritins on erythroid cells. Am J Hematol. 1992 Apr;39(4):264–268. doi: 10.1002/ajh.2830390406. [DOI] [PubMed] [Google Scholar]
- Fargion S., Fracanzani A. L., Brando B., Arosio P., Levi S., Fiorelli G. Specific binding sites for H-ferritin on human lymphocytes: modulation during cellular proliferation and potential implication in cell growth control. Blood. 1991 Aug 15;78(4):1056–1061. [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987 Feb 15;161(1):207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Geerts A., Lazou J. M., De Bleser P., Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991 Jun;13(6):1193–1202. [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Kondo H., Saito K., Grasso J. P., Aisen P. Iron metabolism in the erythrophagocytosing Kupffer cell. Hepatology. 1988 Jan-Feb;8(1):32–38. doi: 10.1002/hep.1840080108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mack U., Powell L. W., Halliday J. W. Detection and isolation of a hepatic membrane receptor for ferritin. J Biol Chem. 1983 Apr 25;258(8):4672–4675. [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Moss D., Fargion S., Fracanzani A. L., Levi S., Cappellini M. D., Arosio P., Powell L. W., Halliday J. W. Functional roles of the ferritin receptors of human liver, hepatoma, lymphoid and erythroid cells. J Inorg Biochem. 1992 Aug 15;47(3-4):219–227. doi: 10.1016/0162-0134(92)84067-w. [DOI] [PubMed] [Google Scholar]
- Moss D., Powell L. W., Arosio P., Halliday J. W. Characterization of the ferritin receptors of human T lymphoid (MOLT-4) cells. J Lab Clin Med. 1992 Mar;119(3):273–279. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nichols G. M., Bacon B. R. Hereditary hemochromatosis: pathogenesis and clinical features of a common disease. Am J Gastroenterol. 1989 Aug;84(8):851–862. [PubMed] [Google Scholar]
- Ono T., Seno S. Transport of ferritin from Kupffer cells to liver parenchymal cells. Morphological and immunocytochemical observations. Int J Hematol. 1991 Apr;54(2):93–102. [PubMed] [Google Scholar]
- Pietrangelo A., Gualdi R., Casalgrandi G., Geerts A., De Bleser P., Montosi G., Ventura E. Enhanced hepatic collagen type I mRNA expression into fat-storing cells in a rodent model of hemochromatosis. Hepatology. 1994 Mar;19(3):714–721. doi: 10.1002/hep.1840190325. [DOI] [PubMed] [Google Scholar]
- Pollack S., Campana T. Immature red cells have ferritin receptors. Biochem Biophys Res Commun. 1981 Jun;100(4):1667–1672. doi: 10.1016/0006-291x(81)90710-5. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Veit T., Schwögler S., Dienes H. P., Knittel T., Rieder H., Meyer zum Büschenfelde K. H. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Sibille J. C., Kondo H., Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology. 1988 Mar-Apr;8(2):296–301. doi: 10.1002/hep.1840080218. [DOI] [PubMed] [Google Scholar]
- Stefanini S., Chiancone E., Arosio P., Finazzi-Agrò A., Antonini E. Structural heterogeneity and subunit composition of horse ferritins. Biochemistry. 1982 May 11;21(10):2293–2299. doi: 10.1021/bi00539a004. [DOI] [PubMed] [Google Scholar]
- Takahara T., Kojima T., Miyabayashi C., Inoue K., Sasaki H., Muragaki Y., Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988 Oct;59(4):509–521. [PubMed] [Google Scholar]
- Takami M., Mizumoto K., Kasuya I., Kino K., Sussman H. H., Tsunoo H. Human placental ferritin receptor. Biochim Biophys Acta. 1986 Oct 29;884(1):31–38. doi: 10.1016/0304-4165(86)90223-0. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Cooper S., Broxmeyer H. E. Effects of hematopoietic suppressor molecules on the in vitro proliferation of purified murine granulocyte-macrophage progenitor cells. Cancer Res. 1988 Mar 15;48(6):1548–1550. [PubMed] [Google Scholar]
- de Leeuw A. M., McCarthy S. P., Geerts A., Knook D. L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984 May-Jun;4(3):392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]