Abstract
Uncx (Phd1, Chx4) is a paired homeobox transcription factor gene. It and its probable functional partners, Tle co-repressors, were expressed by neurally-fated basal progenitor cells and olfactory sensory neurons of the olfactory epithelium. Uncx expression was rare in olfactory epithelia of Ascl1−/− mice, but common in Neurog1−/− mice. In Uncx−/− mice olfactory progenitor cell proliferation, progenitor cell number, olfactory sensory neuron survival, and Umodl1 and Kcnc4 mRNAs were reduced. Evidence of sensory neuron activity and functional connections to the olfactory bulb argue that decreased neuronal survival was not due to loss of trophic support or activity-dependent mechanisms. These data suggest that UNCX acts downstream of neural determination factors to broadly control transcriptional mechanisms used by neural progenitor cells to specify neural phenotypes.
Keywords: Tle3, Umodl1, Kcnc4, neural development, neurogenesis, olfaction
Introduction
Uncx (Unx4.1, Chx4, Phd1) is a paired homeobox transcription factor gene. It is a homolog of the Caenorhabditis elegans Unc-4, whose encoded protein acts to specify the synaptic choices made by one type of motor neuron via partnering with UNC-37, a homolog of Gro/TLE transcriptional co-repressors (Miller and Niemeyer, 1995; Von Stetina et al., 2007; Winnier et al., 1999). In mammalian development, Uncx is best known for expression in the caudal half of new somites where it is necessary for proper formation of the axial skeleton (Leitges et al., 2000; Mansouri et al., 2000). However, it is also expressed in the developing central nervous system (Leitges et al., 2000; Mansouri et al., 1997). Uncx is expressed in neural progenitor cells of the dorsal neural tube in regions that give rise to the spinal cord and regions of the telencephalon (Saito et al., 1996). However, the mouse tissue with the highest level of Uncx mRNA is the olfactory epithelium (GeneAtlas GNF1M dataset, http://biogps.gnf.org). In the embryonic epithelium, Uncx is expressed in olfactory sensory neurons (OSNs) and in basal progenitor cells, where it may be directly regulated by the neural determination factor, Ascl1 (Mash1) (Cau et al., 2002; Saito et al., 1996). The continuous neurogenesis that occurs in the olfactory epithelium provides an advantageous system to test the long-standing hypothesis (Saito et al., 1996) that Uncx acts downstream of basic helix-loop-helix neural determination factors to control neurogenesis. Herein we describe results of tests of whether Uncx is essential for development and homeostasis of OSNs.
The development of OSNs is a lifelong process whereby proliferating basal cells continuously replace OSNs, presumably because the OSNs become damaged in their uniquely exposed location. The control of gene transcription in the OSN cell lineage is complex. OSNs alone express more than 200 transcriptional regulators, not counting the chromatin remodeling genes that are also overrepresented among the ~10,000 genes they express (Sammeta et al., 2007). Factors that regulate gene expression in the progenitor cells of the OSN cell lineage have not been so broadly described, but several critical transcription factors are known. Foxg1, Six1, and Six4 are necessary for embryonic neurogenesis in the olfactory placode (Chen et al., 2009; Duggan et al., 2008; Ikeda et al., 2007; Kawauchi et al., 2004). Ascl1 is necessary for the neural fate decision for nearly all OSNs (Guillemot et al., 1993). The specification and differentiation of OSNs depends in part on Lhx2, Zfp423 (OAZ), and Runx1 (Cau et al., 2002; Cheng and Reed, 2007; Hirota and Mombaerts, 2004; Kolterud et al., 2004; Theriault et al., 2005). Less profound defects in OSNs result from the absence of Neurog1, Emx2, Dlx5, Klf7, Fezf1, or the Ebf transcription factors (Cau et al., 2002; Cau et al., 2000; Hirata et al., 2006; Kajimura et al., 2007; Laub et al., 2005; Levi et al., 2003; McIntyre et al., 2008; Wang et al., 2004; Wang et al., 1997).
Whether Uncx also has irreplaceable roles in the OSN cell lineage was unknown. We found that Uncx was required for normal levels of basal cell proliferation. In addition, survival of OSNs was reduced in the absence of Uncx.
Results
Uncx is expressed throughout the olfactory sensory neuron lineage
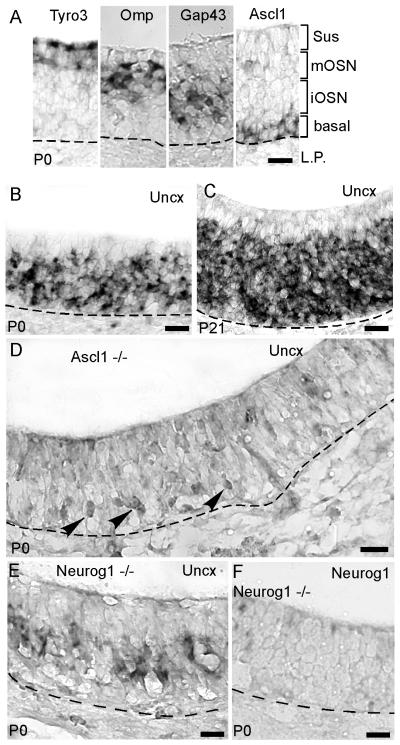
As expected from studies of mouse embryos and neonatal rats (Cau et al., 2002; Saito et al., 1996), we observed that Uncx was a highly abundant mRNA in the postnatal mouse olfactory epithelium (Fig. 1B,C). In situ hybridization detected Uncx mRNA in both mature and immature OSN layers, as well as in the basal cell layer. We did not detect Uncx in sustentacular cells, horizontal basal cells, or in cells of the lamina propria. This expression pattern is consistent with Uncx mRNA localization in the embryonic and early neonatal olfactory epithelium (Cau et al., 2002; Saito et al., 1996), indicating that Uncx expression is a developmentally consistent feature of the OSN cell lineage. To verify that Uncx expression labeled OSNs and basal cells of the OSN cell lineage we turned to Ascl1−/− mice, in which the neural fate decision is blocked and no OSNs or immediate neuronal precursor cells form (Cau et al., 2002; Guillemot et al., 1993). As observed previously in Ascl1−/− mouse embryos, Uncx was absent at birth except perhaps for a few basally located cells (Fig. 1D). Previous studies have proposed that these faintly labeled cells have managed to transition toward a neural fate even in the absence of Ascl1 (Cau et al., 2002; Guillemot et al., 1993). If Uncx expression is dependent (even indirectly) on Ascl1, then it may be less sensitive to the loss of transcription factors that regulate later stages of the OSN cell lineage. Neurog1 (Ngn1), which is thought to be involved in neural specification of OSNs, is expressed in immediate neuronal precursor cells of the OSN lineage downstream of the globose basal cells that express Ascl1 (Calof and Chikaraishi, 1989; Cau et al., 2002; DeHamer et al., 1994; Gordon et al., 1995). In Neurog1−/− mice, which do produce OSNs, we detected Uncx expression (Fig 1E). These findings argue that Uncx expression is not wholly dependent on Neurog1, that Uncx is expressed in all cell types in the OSN lineage subsequent to the determination of neural fate, and that Uncx expression may even begin within the transit amplifying cells that express Ascl1.
Fig. 1.
Uncx is expressed in all cells of the OSN lineage. (A) A guide to the cell layers of the olfactory epithelium at age P0 using in situ hybridization for cell-type specific markers: Tyro3 for sustentacular cells, Omp for mature OSNs, Gap43 for immature OSNs, and Ascl1 (Mash1) for basal cells. (B,C) In situ hybridization for Uncx in C57Bl6 mice at ages P0 and P21. (D) In situ hybridization for Uncx in Ascl1−/− mice at age P0 faintly labels a few cells. (E) In situ hybridization for Uncx in Neurog1−/− mice at age P0. (F) Negative control: in situ hybridization for Neurog1 in Neurog1−/− mice at age P0. Dashed lines, basal lamina. Scale bars, 20 μm.
Absence of Uncx leads to fewer mature OSNs
To further investigate the role of Uncx in the OSN lineage we analyzed Uncx−/− mice. In these mice the morphologies of the anterior head structures and the nasal cavity appeared normal. The respiratory epithelium in the nasal cavity also appeared normal but there was a marked decrease in the thickness of the olfactory epithelium from 84 ± 3 μm to 68 ± 4 μm (t = 7.0574, p = 0.0001, n = 5 mice). A decrease in thickness is usually caused by a reduction in the number of OSNs, which constitute the majority of cells in the epithelium. As predicted, in situ hybridization and immunohistochemistry detected a 52% reduction in OMP+ mature OSNs in age P0 Uncx−/− mice (Table 1; Fig. 2A, B). OMP+ mature OSNs were present at embryonic day 14.5 in the absence of Uncx (Fig. 2C, D), indicating that the lack of Uncx did not prevent the embryonic development of mature OSNs. Overall, the data suggest that the production and survival of OSNs might be reduced in Uncx−/− mice.
Table 1.
Cell counts per 0.1 mm width of olfactory epithelium.
| Marker | Uncx−/− (mean ± SD) |
Uncx+/− (mean ± SD) |
t-statistic | p-value |
|---|---|---|---|---|
| Omp (P0) | 13.1 ± 1.3 | 26.9 ± 4.5 | −5.06675 | 0.00357 |
| Gap43 (P0) | 17.6 ± 1.6 | 24.6 ± 2.0 | −4.69757 | 0.00466 |
| Ascl1 (P0) | 4.4 ± 0.6 | 7.6 ± 0.4 | −7.81419 | 0.00072 |
| Ascl1 (E14.5) | 8.7 ± 2.5 | 14.2 ± 2.1 | −2.9174 | 0.04335 |
| Casp3 (P0) | 1.1 ± 0.2 | 0.4 ± 0.1 | 5.28902 | 0.00613 |
| phosphoH3 (P0) | 1.4 ± 0.2 | 2.5 ± 0.4 | −4.09878 | 0.01487 |
| Ccnd1 (P0) | 6.8 ± 0.3 | 9.1 ± 1.0 | −3.71585 | 0.02055 |
Omp, mature OSNs; Gap43, immature OSNs; Ascl1, transit amplifying basal progenitor cells; Casp3, active fragment of caspase-3 to identify apoptosis; phosphoH3, phosphorylated histone-3 to identify mitosis; Ccnd1, cyclin D1. N = 3 mice in each case. Ages: postnatal day 0 (P0) and embryonic day 14.5 (E14.5).
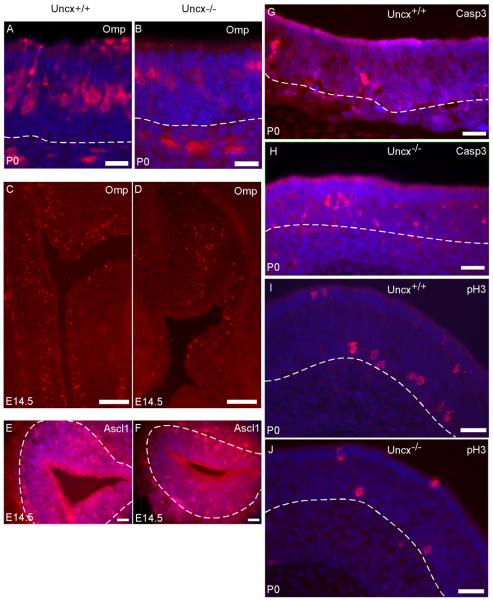
Fig. 2.
The absence of Uncx increases OSN apoptosis and reduces basal cell proliferation. Mature OSNs appeared to be less abundant in Uncx−/− mice according to immunofluorescence for OMP protein (A – D). (E, F) Cells immunoreactive for ASCL1 appeared to be less abundant in Uncx−/− mice at age E14.5. (G, H) Caspase 3 immunoreactivity in the OSN layer appeared to be increased in Uncx−/− mice. (I, J) Fewer cells appeared to be immunoreactive for phosphorylated histone-3 in Uncx−/− mice. Blue: Hoechst labeling of nuclei. Scale bars: A-F, I-N, 20 μm; G, H, 50 μm.
Uncompensated increase in OSN apoptosis
To determine if the reduction in the mature OSNs was due to increased apoptosis, we did immunohistochemistry for the active fragment of caspase-3, an indicator of OSN apoptosis (Carson et al., 2005; Cowan et al., 2001). Nearly all (88%) immunoreactive cells detected were located in the OSN layers of the olfactory epithelium, irrespective of genotype (Fig. 2G, H). Quantification revealed a 2.6-fold increase in the number of apoptosing OSNs in Uncx−/− mice compared to wild type littermates (Table 1). The absence of Uncx, therefore, increased the rate of OSN apoptosis, thereby contributing to a reduction in the number of OSNs present.
Increased apoptosis of OSNs typically leads to an increase in the proliferation of basal progenitor cells (Schwob, 2002). We assessed proliferation by immunohistochemistry for phosphorylated histone-3 to label mitotic cells. Contrary to the normal reaction of the epithelium to a reduction in mature OSNs, the epithelia of Uncx−/− mice showed 42% fewer mitotic cells in the basal cell layer than did wild type littermates (Fig. 2I, J; Table 1). Consistent with this observation, we also detected 26% fewer cells expressing Ccnd1 (Fig. 3A,B; Table 1), a cell cycle mRNA known to increase when basal cell proliferation increases in the olfactory epithelium (Shetty et al., 2005). These findings indicate that the loss of Uncx reduces proliferation of basal cell progenitors in the OSN lineage even during increased OSN cell death.
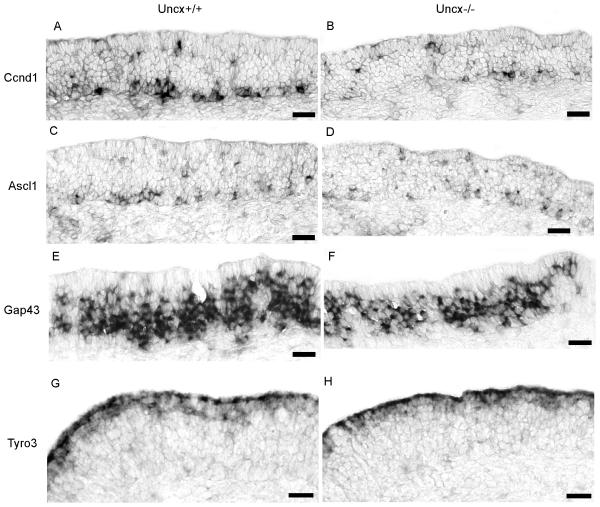
Fig. 3.
Fewer basal cells and immature OSNs were apparent in Uncx−/− mice. (A, B) In situ hybridization for Ccnd1 to label proliferating basal cells detected fewer cells in Uncx−/− mice. (C, D) In situ hybridization for Ascl1 to label transit amplifying basal cells detected fewer cells in Uncx−/− mice. (E, F) In situ hybridization for Gap43 to label immature OSNs detected fewer cells in Uncx−/− mice. (G, H) In situ hybridization for Tyro3 to label sustentacular cells was unaffected by the absence of Uncx. Scale bars, 20 μm.
We tested whether transcripts for Bcl2 and Bid, key regulators of OSN apoptosis, were altered by the absence of Uncx. Bid mRNA, which encodes a pro-apoptotic protein, was unaffected but the Bcl2 mRNA, which encodes an anti-apoptotic protein, was elevated 25% in olfactory epithelia of mice lacking Uncx (t = 3.1509, p = 0.0077, df = 13).
Decreased neurogenesis in the absence of Uncx
Decreased basal cell proliferation in Uncx−/− mice suggests that in addition to a reduction in mature OSNs the olfactory epithelium has fewer cells at each stage in the OSN lineage when Uncx is absent. We therefore quantified other cell types in the OSN lineage. At birth and at embryonic day E14.5, Uncx−/− mice had fewer Ascl1+ transit amplifying progenitors (Fig. 2E,F; Fig. 3C,D; Table 1). They also had 28% fewer Gap43+ immature OSNs (Fig. 3E, F; Table 1). These data argue that Uncx is necessary to support normal rates of olfactory neurogenesis.
One mechanism by which the absence of Uncx could affect olfactory neurogenesis is to alter the balance between Gdf11 and follistatin. Gdf11, expressed primarily in olfactory progenitor cells, inhibits basal cell proliferation while follistatin, expressed primarily in cells of the underlying lamina propria, stimulates proliferation by inhibiting Gdf11 (Kawauchi et al., 2009; Wu et al., 2003). However, we did not detect altered amounts of Gdf11 and follistatin mRNAs in olfactory epithelia of Uncx−/− mice (t = 0.02, p = 0.98 and t = 0.53, p = 0.61, respectively, df = 13). This finding argues that altered Gdf11/follistatin signaling cannot explain reduced neurogenesis in Uncx−/− mice.
Sustentacular cell density and survival is unaffected by the loss of Uncx
One mechanism by which Uncx could contribute to olfactory neurogenesis is by cooperating with Ascl1 in cell fate determination. The absence of Uncx might then lead to diversion of a subset of early progenitors into an alternative fate. The most likely alternative fate would be the sustentacular cells, glial-like cells that form the apical surface of the epithelium and surround the OSNs. However, we observed no increase in the number of sustentacular cells (Fig 3G,H), nor did we notice either a compensating increase in apoptosis in the sustentacular cell layer via labeling for active caspase-3 or a compensating decrease in mitosis within the sustentacular cell layer.
Uncx−/− OSNs make functional connections in the olfactory bulb
To begin to test whether the OSNs produced in Uncx−/− mice could be functionally normal, we first observed their axonal projections to the olfactory bulb (Fig. 4A-D). The overall organization of the OSN nerve layer and glomeruli was similar in Uncx−/− and wild type mice.
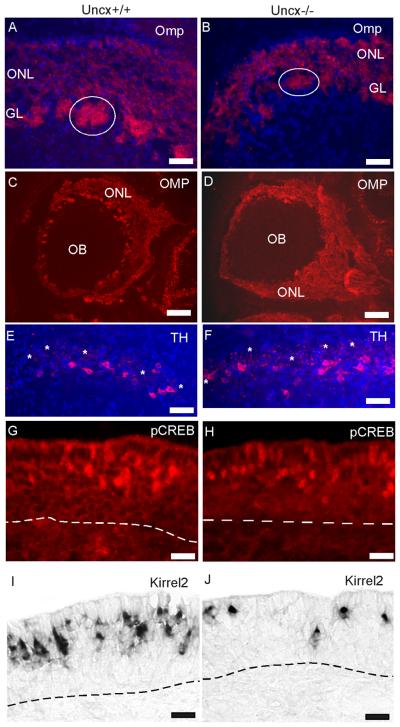
Fig. 4.
Evidence of OSN activity and functional connections to the olfactory bulb (OB) in Uncx−/− mice. (A - D) Detection of OSN axons by OMP immunoreactivity revealed an olfactory nerve layer (ONL) and innervation of glomeruli (e.g, circles) in Uncx−/− mice that were positioned normally. (E, F) Immunoreactivity for the activity marker, tyrosine hydroxylase (TH), detected similar numbers of periglomerular neurons in the olfactory bulbs of Uncx−/− and Uncx+/+ mice. Some glomeruli are marked in the images (asterisks). (G - J) Two activity markers of OSNs, phosphorylated CREB (pCREB) and Kirrel2 were detected in OSNs of Uncx−/− mice, though they appeared to be fewer. Kirrel2 expression was detected by in situ hybridization, pCREB by immunofluorescence. Blue, Hoechst 33342 nuclear stain, Dashed lines, basal lamina; GL, glomerular layer. Scale bars: A, B, E, F, 50 μm; C, D, 500 μm; G - J, 20 μm.
We also assessed whether the OSNs of Uncx−/− mice could be active. We observed normal patterns of odorant receptor expression in OSNs, normal patterns of immunoreactivity for ADCY3 in the cilia layer above the epithelium, and normal identification of OSN dendritic knobs by CROCC, a component of the basal body of OSN cilia (see supplemental data) (McClintock et al., 2008). These indications of a capacity to respond to odors led to tests of expression of activity markers. Tyrosine hydroxylase expression in the periglomerular neurons of the olfactory bulb is controlled by OSN activity (Baker et al., 1983; Nadi et al., 1981). We detected robust expression of tyrosine hydroxylase in periglomerular neurons of the olfactory bulb, an indication that OSNs make functional connections to the bulb in Uncx−/− mice (Fig. 4E,F; supplemental data). We further confirmed OSN activity by detecting phosphorylated CREB immunoreactivity in OSNs (Fig. 4G,H), an even more direct indicator (Hagglund et al., 2006). In addition, we observed robust expression of Kirrel2, an activity-dependent gene in OSNs (Serizawa et al., 2006), in Uncx−/− mice (Fig. 4I, J). Importantly, both CREB immunoreactivity and Kirrel2 expression were the same mosaic patterns that occur in wild-type mice where odor stimulation is the major source of OSN activity.
Altered expression of Kcnc4 and Umodl in OSNs that lack Uncx
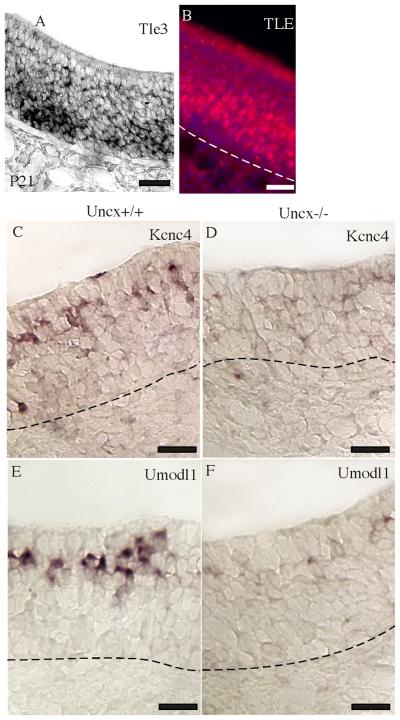
If UNCX functions similarly to its invertebrate homologs, it works through the TLE family of transcriptional co-repressors (Miller and Niemeyer, 1995; Winnier et al., 1999). To determine whether UNCX has access to TLE proteins in OSNs, we confirmed expression of Tle3 mRNA in OSNs and basal cells (Leon and Lobe, 1997; Sammeta et al., 2007) and then determined that Tle proteins are also present in these cells. The intensity of labeling for both mRNA and protein was greatest in the basal half of the neuronal layer of the olfactory epithelium (Fig. 5A - B), occupied by immature OSNs (Fig. 1A).
Fig. 5.
Expression of Tle, Umodl1, and Kcnc4. (A) In situ hybridization detected Tle3 mRNA primarily in the immature OSN and basal cell layers of the olfactory epithelium. (B) A pan-TLE antibody detected TLE proteins primarily in the immature OSN layer. (C - F) Kcnc4 and Umodl1 mRNAs were abundant in Uncx+/+ mature OSNs but difficult to detect in Uncx−/− mature OSNs. Scale bars, 20 μm.
Given that UNCX and its putative partners, TLE proteins, are transcription factors we should detect altered gene expression in OSNs and their progenitors in Uncx−/− mice. Indeed, we found that the mRNAs for Kcnc4, a potassium channel subunit, and Umodl1 (N8), a secreted cell adhesion-like protein, were decreased in Uncx−/− mice (Fig. 5C-F). Both of these mRNAs are expressed specifically in mature OSNs (Sammeta et al., 2007; Yu et al., 2005). The degree of reduction of these mRNAs was disproportionately greater than the 52% reduction in the number of mature OSNs in Uncx−/− mice. Kcnc4 mRNA was reduced 4.5-fold (t = 5.19, p = 0.0013, df = 6). Umodl1 mRNA was reduced 3.9-fold (t = 4.54, p = 0.00274, df = 6).
Discussion
Uncx expression was detected in the cells of the OSN lineage, from basal progenitor cells to mature OSNs. UNCX proved to be necessary for normal levels of olfactory neurogenesis, specifically for maintaining basal cell proliferation and for survival of OSNs. The OSNs of Uncx−/− mice showed evidence of activity and functional connections with neurons of the olfactory bulb, indicating that these OSNs were either functionally normal or nearly so. Signaling events known to regulate olfactory neurogenesis and survival in vivo (Gdf11/follistatin, trophic support from the bulb, activity dependent survival) appeared normal in Uncx−/− mice, so these results are more consistent with cell-autonomous defects in the OSN lineage of Uncx−/− mice than with changes in external factors. The data argue that Uncx stimulates the proliferation of basal progenitor cells either directly or by facilitating the specification of cell types that proliferate. Similarly, increased OSN apoptosis in the absence of Uncx might indicate that Uncx contributes directly to apoptotic control or that Uncx makes broad contributions to the specification of the OSN phenotype that are necessary for cell homeostasis.
We suspect that a neural specification function is the general role for UNCX in mammalian neural tissues, consistent with evidence that Uncx and its nonmammalian homologs participate in the specification and differentiation of other cell types (Buscarlet and Stifani, 2007; Gasperowicz and Otto, 2005; Miller and Niemeyer, 1995; Winnier et al., 1999). The expression pattern of Uncx in the mouse central nervous system is potentially consistent with specification functions. Uncx is expressed there only during development, where it is limited to neurally-fated progenitor cells in certain regions of the neural tube (Saito et al., 1996). UNCX also appears to have molecular functions that would allow it to broadly regulate gene expression, a property useful for specification functions. It probably partners with TLE co-repressors given that UNCX contains Eh1 domains known to bind TLE proteins and that nonmammalian homologs of UNCX work through TLE homologs (Buscarlet and Stifani, 2007; Copley, 2005; Gasperowicz and Otto, 2005; Swingler et al., 2004). TLE proteins act broadly through chromatin modification mechanisms to regulate cell fate decisions and differentiation (Sekiya and Zaret, 2007; Sierra et al., 2006). If neural specification is indeed the function of UNCX its continued expression into differentiated OSNs is unusual, but OSNs are also unusual in that they evolved to apoptose, a feature that proved to be sensitive to the absence of Uncx.
UNCX acts in all cells with a neural fate in the OSN lineage
Uncx is expressed in basal progenitor cells (Saito et al., 1996). We confirmed that a few Uncx expressing cells remain in Ascl1−/− mice and we concur with the previously proposed explanation that low levels of Uncx expression might arise in a few cells that begin an abortive progression toward a neural fate (Guillemot et al., 1993; Saito et al., 1996). We also found that in the absence of Uncx, fewer Ascl1 positive cells exist. These observations suggest that Uncx expression is triggered immediately by the neural fate decision in basal progenitor cells. This is consistent with the ability of chicken Uncx to be induced by intrinsic fate decisions within presomitic mesoderm (Schragle et al., 2004), and with Notch signaling control of Uncx expression in mouse somitic mesoderm (Sewell et al., 2009). Whether Uncx transcription is driven directly by Ascl1 or by independent mechanisms is uncertain, but once the neural fate decision is made Uncx expression is continued in all subsequent cell types in the OSN lineage.
Basal cell proliferation and OSN survival in the absence of UNCX
The response of basal progenitor cells to the absence of UNCX was reduced proliferation. This defect was not due to increased basal cell apoptosis. Perhaps UNCX regulates a signal that acts upon the basal cells to enhance their proliferation. Tests of a known signal of this type, the Gdf11/follistatin signaling system, did not detect changes, however. A more parsimonious explanation is that basal cells depend on Uncx to stimulate their progression through the cell cycle, perhaps by contributing to the transcriptional regulation of basal cell phenotypes, such as the expression of other transcription factors or of cell cycle control genes.
The reduction in basal cell proliferation in Uncx−/− mice slowed the rate of production of immature OSNs. This contributed to a 28% reduction in Gap43+ immature OSNs and, along with increased apoptosis of OSNs, resulted in a 52% reduction in mature OSNs. The mature OSNs that do arise in Uncx−/− mice make functional synaptic connections in the olfactory bulb and express activity-dependent markers. Our data do not support explanations for increased OSN apoptosis that involve loss of trophic support from the bulb or reduced activity of OSNs (Cowan et al., 2001; Hayward et al., 2004; Robinson et al., 2003; Sultan-Styne et al., 2009).
The evidence implicating UNCX in neural specification, though circumstantial, raises the possibility that increased apoptosis of OSNs in the absence of UNCX results from specification defects. This hypothesis would require that while UNCX might not be uniquely responsible for the regulation of any critical gene, its absence would be sufficient to cause minor imbalances in the networks of proteins that make up the OSN phenotype and thereby decrease the ability of OSNs to maintain homeostasis. Alternatively, UNCX might be directly involved in the control OSN apoptosis. However, we found no evidence that the absence of UNCX shifts the balance of apoptotic factors toward expression of pro-apoptotic genes. The available data do not yet rule out either hypothesis but do point toward further study of the repression of apoptosis genes and basal cell-specific genes by Uncx.
Oldest OSNs most affected by the absence of UNCX?
At present we have identified only two mRNAs whose abundance was affected disproportionately to changes in the number of OSNs in the absence of Uncx: Umodl1 and Kcnc4. While it is possible that these genes are regulated by Uncx, we are skeptical of this explanation. We have previously demonstrated that Umodl1 mRNA is specific to mature OSNs and that its abundance increases along a gradient from basal to apical across the mature OSN layer (Yu et al., 2005). Kcnc4, the other mRNA whose abundance was disproportionately reduced in Uncx−/− mice, showed the same gradient of abundance within the mature OSN layer. If position within the olfactory epithelium controlled expression of these genes, then they should not have been so sensitive to the loss of Uncx. However, early studies of mammalian olfactory anatomy suggest that increased OSN age may correlate with apical position (Graziadei and Monti Graziadei, 1978). If so, then the most apically located OSNs would be the oldest. If OSN age, or an age-related factor such as damage from chemical and pathogen exposure, stimulates expression of Umodl1 and Kcnc4 then the increased rate of OSN apoptosis we observed probably shortens the average lifespan of OSNs, potentially explaining the disproportionate reduction in Umodl1 and Kcnc4 transcripts. This idea is consistent with evidence that Kcnc4 expression is sensitive to neuronal damage - specifically that Kcnc4 expression increases in neurodegenerative structures during Alzheimer's disease (Angulo et al., 2004). The neonatal lethality of Uncx−/− mice has impeded our ability to directly test whether their OSNs are more sensitive to damage.
Experimental Procedures
Mice
Mice carrying a targeted deletion of Uncx were obtained from Dr. Ahmed Mansouri (Max-Planck Institute for Biophysical Chemistry, Gottingen, Germany) (Mansouri et al., 2000). Uncx−/− mice die within the first day of life, so only newborn and embryonic mice were used for experiments testing the effects of the absence of Uncx. Mice carrying a targeted deletion of Ascl1 were obtained from Dr. Randall Reed (Johns Hopkins University, Baltimore, MD). Mice carrying a targeted deletion of Neurog1 were obtained from Dr. David Anderson, California Institute of Technology, Pasadena, CA). Except where indicated, all samples were obtained from mice less than 24 hours old, postnatal day 0 (P0). Mice were housed in the animal facility at the University of Kentucky. All procedures with mice were done according to approved Institutional Animal Care and Use Committee protocols and conformed to NIH guidelines.
Genes and Uncx cDNA
Mouse genes and mRNAs are displayed according to the gene symbol conventions of Entrez Gene (National Center for Biotechnology Information), and the gene symbol is capitalized when referring to the protein product of the gene. To avoid ambiguity, the Entrez Gene IDs, gene names, gene symbols, and common synonyms for all mouse genes mentioned herein are listed in Supplemental Table S1.
In situ hybridization and immunohistochemistry
In situ hybridization was done on 10 μm coronal cryosections of the anterior head as described previously (Sammeta et al., 2007). For each gene, cDNA fragments (400-500 bp) were amplified by PCR from olfactory epithelium cDNA and cloned into pBluescript. The primer positions are listed in Supplemental Table S1. The fragments chosen were selected to have less than 80% identity to any other mouse gene. Sense and antisense recombinant RNA probes (approximately 500 bp in length) labeled with digoxygenin were prepared and used for hybridization against the tissue sections. Sense controls were invariably negative. A detailed protocol, which follows the methods of Ishii and colleagues (Ishii et al., 2003; Ishii et al., 2004), is available from the authors.
Immunohistochemistry was also done on 10 μm coronal cryosections. The frozen sections were thawed and permeabilized in PBS with 1% Triton X-100. Antigen retrieval was done by boiling the slides in 10 mM citrate buffer for 10 min. The slides were allowed to cool to room temperature and incubated for 1h at room temperature in blocking buffer (2% BSA and 0.4% Triton X100 in PBS), followed by overnight incubation in primary antibody diluted in the blocking buffer at 4°C. Sections were washed with 0.05% Tween-20 in PBS for 30 min and then incubated with Cy3 conjugated secondary antibody diluted in PBS. Nuclei were counterstained with Hoechst 33342 (# H1399, Invitrogen, Carlsbad, CA). Primary antibodies were a rabbit polyclonal pan-TLE antiserum (# 4681, Cell Signaling Technology, Danvers, MA) used at a dilution of 1:200, a rabbit polyclonal antibody against the active fragment of cleaved caspase-3 (# 9661S, Cell Signaling Technology Inc.) at a dilution of 1:200, a goat polyclonal OMP antibody (544-10001, Wako Chemicals USA, Richmond, VA) at a dilution of 1:500, a rabbit polyclonal antibodies against phosphorylated histone-3 and phosphorylated CREB (#06-570 and #06-519, Millipore, Billerica, MA) at dilution of 1:200 and 1:1,000, respectively, a rabbit polyclonal antibody against Crocc supplied by Dr. Tiansen Li (Harvard University) at a dilution of 1:5,000, a mouse monoclonal against ASCL1 (MAB2567, R & D Systems, Minneapolis, MN), a rabbit polyclonal against ADCY3 (#sc-588, Santa Cruz Biotechnology, Sant Cruz, CA) at a dilution of 1:200, and a mouse monoclonal antibody against tyrosine hydroxylase (# ab11, Abcam, Cambridge, MA) at a dilution of 1:200. The specificities of these antibodies are documented in publication (Blount et al., 2008; Buiakova et al., 1996; Cheong et al., 2003; Hendzel et al., 1997; Hu et al., 2000; Huang et al., 2007; Kaiser et al., 2008; Rodriguez-Gil and Greer, 2008; Yang et al., 2005; Yang et al., 2002), or via data made available on-line in the case of the pan-TLE antiserum (http://www.cellsignal.com/products/4681.html). A Cy3-conjugated donkey anti-rabbit secondary antibody and a donkey anti-goat antibody (#711-165-152, #705-165-147, Jackson ImmunoReseach) were used at a dilution of 1:1000.
Wide-field images were obtained on a Nikon Diaphot 300 inverted microscope using a Spot 2e camera and Spot software version 4.0.6 through a 40x/0.75 numerical aperture Plan Fluor objective or a 4x/0.13 numerical aperture Plan objective. Images were processed in Adobe Photoshop (version 7.0) by adjusting size and brightness. Images were combined and labeled in Deneba Canvas (version 8.0). The mRNAs and antigens detected for this project are expressed similarly across all regions of the olfactory epithelium, so only high magnification images are used to display their distributions across the layers of this pseudostratified epithelium.
Cell counts were done on images of coronal sections, matched for anterior-posterior position between the genotypes, three mice per genotype. Three sections per mouse were counted at three positions along the epithelium (dorsal recess, dorsal septum, and ventral septum) and normalized to the length of epithelium in each image. Cells were included in the count if their nuclei were contained in the sections. Abercrombie's correction of over-counting of profiles in sections was applied (Abercombie, 1946). Student's t-tests were applied to counts. The observation that the epithelium was thinner implied that fewer cells were present, so one-tailed tests were done for counts of specific cell-types. Counts of markers of proliferation and apoptosis were done as two-tailed tests. Correction for multiple testing was done in cases where more than one marker were tested on the same three mice (Benjamini and Hochberg, 1995).
Quantitative RT-PCR
Reverse transcription was performed on 0.5 μg of total RNA using SuperScript II and random hexamers (Life Technologies, Rockville, MD) in a 50 μl reaction. Primers were designed using Primer Express software (Applied Biosystems, Foster City, CA) and purchased from Integrated DNA Technologies (Coralville, IA). Amplification was performed on triplicate samples in an ABI Prism 7700 Sequence Detection System using 1 μl of the cDNA reaction and the Sybr Green Core Reagent Kit under conditions prescribed by the manufacturer (Applied Biosystems). Thermal cycler conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Whole olfactory epithelium cDNA was used as a template for standard curves, which were required to exceed a criterion correlation coefficient of 0.98 before being accepted for analysis. Melt curves were performed on each sample to verify that each reaction produced a single product. Results were normalized to the geometric mean of four relatively stable common mRNAs in each sample: Gapdh, Actb, Ubc, and Hprt1. Seven heterozygous mice were compared against eight knockout mice using Student's t-test at for α = 0.05. Adjustment of the p-values to correct for unequal variance was necessary for two mRNAs that had large differences in abundance between the genotypes. In addition, correction for multiple testing was done using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) to obtain adjusted p-values for α = 0.05. All data are reported as means ± standard errors of the mean.
Supplementary Material
Acknowledgements
Supported by NIH awards R01 DC002736 and R01 DC007194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Angulo E, Noe V, Casado V, Mallol J, Gomez-Isla T, Lluis C, Ferrer I, Ciudad CJ, Franco R. Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer's disease. J Neurochem. 2004;91:547–557. doi: 10.1111/j.1471-4159.2004.02771.x. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Blount AL, Peled ZM, Dexter EL, Nagle RB, Maloney CT, Dellon AL. Sympathetic nerves in the tarsal tunnel: implications for blood flow in the diabetic foot. Plast Reconstr Surg. 2008;122:188–191. doi: 10.1097/PRS.0b013e31817742c3. [DOI] [PubMed] [Google Scholar]
- Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL, Margolis FL. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A. 1996;93:9858–9863. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscarlet M, Stifani S. The ‘Marx’ of Groucho on development and disease. Trends Cell Biol. 2007;17:353–361. doi: 10.1016/j.tcb.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Carson C, Murdoch B, Roskams AJ. Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn. 2006;235:1678–1688. doi: 10.1002/dvdy.20733. [DOI] [PubMed] [Google Scholar]
- Carson C, Saleh M, Fung FW, Nicholson DW, Roskams AJ. Axonal dynactin p150Glued transports caspase-8 to drive retrograde olfactory receptor neuron apoptosis. J Neurosci. 2005;25:6092–6104. doi: 10.1523/JNEUROSCI.0707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Reed RR. Zfp423/OAZ participates in a developmental switch during olfactory neurogenesis. Neuron. 2007;54:547–557. doi: 10.1016/j.neuron.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JW, Chong SY, Kim JY, Eom JI, Jeung HK, Maeng HY, Lee ST, Min YH. Induction of apoptosis by apicidin, a histone deacetylase inhibitor, via the activation of mitochondria-dependent caspase cascades in human Bcr-Abl-positive leukemia cells. Clin Cancer Res. 2003;9:5018–5027. [PubMed] [Google Scholar]
- Copley RR. The EH1 motif in metazoan transcription factors. BMC Genomics. 2005;6:169. doi: 10.1186/1471-2164-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Thai J, Krajewski S, Reed JC, Nicholson DW, Kaufmann SH, Roskams AJ. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci. 2001;21:7099–7109. doi: 10.1523/JNEUROSCI.21-18-07099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994;13:1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Duggan CD, DeMaria S, Baudhuin A, Stafford D, Ngai J. Foxg1 is required for development of the vertebrate olfactory system. J Neurosci. 2008;28:5229–5239. doi: 10.1523/JNEUROSCI.1134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperowicz M, Otto F. Mammalian Groucho homologs: redundancy or specificity? J Cell Biochem. 2005;95:670–687. doi: 10.1002/jcb.20476. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Monti Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of Sensory Physiology. Springer Verlag; Berlin: 1978. pp. 55–82. [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hagglund M, Berghard A, Strotmann J, Bohm S. Retinoic acid receptor-dependent survival of olfactory sensory neurons in postnatal and adult mice. J Neurosci. 2006;26:3281–3291. doi: 10.1523/JNEUROSCI.4955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Bocchiaro CM, Morgan JI. Expression of Bcl-2 extends the survival of olfactory receptor neurons in the absence of an olfactory bulb. Brain Res Mol Brain Res. 2004;132:221–234. doi: 10.1016/j.molbrainres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hirata T, Nakazawa M, Yoshihara S, Miyachi H, Kitamura K, Yoshihara Y, Hibi M. Zinc-finger gene Fez in the olfactory sensory neurons regulates development of the olfactory bulb non-cell-autonomously. Development. 2006;133:1433–1443. doi: 10.1242/dev.02329. [DOI] [PubMed] [Google Scholar]
- Hirota J, Mombaerts P. The LIM-homeodomain protein Lhx2 is required for complete development of mouse olfactory sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8751–8755. doi: 10.1073/pnas.0400940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Huang MH, Wang HQ, Roeske WR, Birnbaum Y, Wu Y, Yang NP, Lin Y, Ye Y, McAdoo DJ, Hughes MG, Lick SD, Boor PJ, Lui CY, Uretsky BF. Mediating delta-opioid-initiated heart protection via the beta2-adrenergic receptor: role of the intrinsic cardiac adrenergic cell. Am J Physiol Heart Circ Physiol. 2007;293:H376–384. doi: 10.1152/ajpheart.01195.2006. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Ishii T, Hirota J, Mombaerts P. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Curr Biol. 2003;13:394–400. doi: 10.1016/s0960-9822(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Ishii T, Omura M, Mombaerts P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J Neurocytol. 2004;33:657–669. doi: 10.1007/s11068-005-3334-y. [DOI] [PubMed] [Google Scholar]
- Kaiser CL, Chapman BJ, Guidi JL, Terry CE, Mangiardi DA, Cotanche DA. Comparison of activated caspase detection methods in the gentamicin-treated chick cochlea. Hear Res. 2008;240:1–11. doi: 10.1016/j.heares.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura D, Dragomir C, Ramirez F, Laub F. Identification of genes regulated by transcription factor KLF7 in differentiating olfactory sensory neurons. Gene. 2007;388:34–42. doi: 10.1016/j.gene.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Kim J, Santos R, Wu HH, Lander AD, Calof AL. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453–1464. doi: 10.1242/dev.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterud A, Alenius M, Carlsson L, Bohm S. The Lim homeobox gene Lhx2 is required for olfactory sensory neuron identity. Development. 2004;131:5319–5326. doi: 10.1242/dev.01416. [DOI] [PubMed] [Google Scholar]
- Laub F, Lei L, Sumiyoshi H, Kajimura D, Dragomir C, Smaldone S, Puche AC, Petros TJ, Mason C, Parada LF, Ramirez F. Transcription factor KLF7 is important for neuronal morphogenesis in selected regions of the nervous system. Mol Cell Biol. 2005;25:5699–5711. doi: 10.1128/MCB.25.13.5699-5711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Neidhardt L, Haenig B, Herrmann BG, Kispert A. The paired homeobox gene Uncx4.1 specifies pedicles, transverse processes and proximal ribs of the vertebral column. Development. 2000;127:2259–2267. doi: 10.1242/dev.127.11.2259. [DOI] [PubMed] [Google Scholar]
- Leon C, Lobe CG. Grg3, a murine Groucho-related gene, is expressed in the developing nervous system and in mesenchyme-induced epithelial structures. Dev Dyn. 1997;208:11–24. doi: 10.1002/(SICI)1097-0177(199701)208:1<11::AID-AJA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Levi G, Puche AC, Mantero S, Barbieri O, Trombino S, Paleari L, Egeo A, Merlo GR. The Dlx5 homeodomain gene is essential for olfactory development and connectivity in the mouse. Mol Cell Neurosci. 2003;22:530–543. doi: 10.1016/s1044-7431(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Voss AK, Thomas T, Yokota Y, Gruss P. Uncx4.1 is required for the formation of the pedicles and proximal ribs and acts upstream of Pax9. Development. 2000;127:2251–2258. doi: 10.1242/dev.127.11.2251. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Yokota Y, Wehr R, Copeland NG, Jenkins NA, Gruss P. Paired-related murine homeobox gene expressed in the developing sclerotome, kidney, and nervous system. Dev Dyn. 1997;210:53–65. doi: 10.1002/(SICI)1097-0177(199709)210:1<53::AID-AJA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Glasser CE, Bose SC, Bergman DA. Tissue expression patterns identify mouse cilia genes. Physiol Genomics. 2008;32:198–206. doi: 10.1152/physiolgenomics.00128.2007. [DOI] [PubMed] [Google Scholar]
- McIntyre JC, Bose SC, Stromberg AJ, McClintock TS. Emx2 stimulates odorant receptor gene expression. Chem Senses. 2008;33:825–837. doi: 10.1093/chemse/bjn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, 3rd, Niemeyer CJ. Expression of the unc-4 homeoprotein in Caenorhabditis elegans motor neurons specifies presynaptic input. Development. 1995;121:2877–2886. doi: 10.1242/dev.121.9.2877. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–377. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Robinson AM, Conley DB, Kern RC. Olfactory neurons in bax knockout mice are protected from bulbectomy-induced apoptosis. Neuroreport. 2003;14:1891–1894. doi: 10.1097/00001756-200310270-00002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Greer CA. Wnt/Frizzled family members mediate olfactory sensory neuron axon extension. J Comp Neurol. 2008;511:301–317. doi: 10.1002/cne.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Lo L, Anderson DJ, Mikoshiba K. Identification of novel paired homeodomain protein related to C. elegans unc-4 as a potential downstream target of MASH1. Dev Biol. 1996;180:143–155. doi: 10.1006/dbio.1996.0291. [DOI] [PubMed] [Google Scholar]
- Sammeta N, Yu TT, Bose SC, McClintock TS. Mouse olfactory sensory neurons express 10,000 genes. J Comp Neurol. 2007;502:1138–1156. doi: 10.1002/cne.21365. [DOI] [PubMed] [Google Scholar]
- Schragle J, Huang R, Christ B, Prols F. Control of the temporal and spatial Uncx4.1 expression in the paraxial mesoderm of avian embryos. Anat Embryol (Berl) 2004;208:323–332. doi: 10.1007/s00429-004-0404-3. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Zaret KS. Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo. Mol Cell. 2007;28:291–303. doi: 10.1016/j.molcel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Sewell W, Sparrow DB, Smith AJ, Gonzalez DM, Rappaport EF, Dunwoodie SL, Kusumi K. Cyclical expression of the Notch/Wnt regulator Nrarp requires modulation by Dll3 in somitogenesis. Dev Biol. 2009;329:400–409. doi: 10.1016/j.ydbio.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty RS, Bose SC, Nickell MD, McIntyre JC, Hardin DH, Harris AM, McClintock TS. Transcriptional changes during neuronal death and replacement in the olfactory epithelium. Mol Cell Neurosci. 2005;30:90–107. doi: 10.1016/j.mcn.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan-Styne K, Toledo R, Walker C, Kallkopf A, Ribak CE, Guthrie KM. Long-term survival of olfactory sensory neurons after target depletion. J Comp Neurol. 2009;515:696–710. doi: 10.1002/cne.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler TE, Bess KL, Yao J, Stifani S, Jayaraman PS. The proline-rich homeodomain protein recruits members of the Groucho/Transducin-like enhancer of split protein family to co-repress transcription in hematopoietic cells. J Biol Chem. 2004;279:34938–34947. doi: 10.1074/jbc.M404488200. [DOI] [PubMed] [Google Scholar]
- Theriault FM, Nuthall HN, Dong Z, Lo R, Barnabe-Heider F, Miller FD, Stifani S. Role for Runx1 in the proliferation and neuronal differentiation of selected progenitor cells in the mammalian nervous system. J Neurosci. 2005;25:2050–2061. doi: 10.1523/JNEUROSCI.5108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina SE, Fox RM, Watkins KL, Starich TA, Shaw JE, Miller DM., 3rd UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes Dev. 2007;21:332–346. doi: 10.1101/gad.1502107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Lewcock JW, Feinstein P, Mombaerts P, Reed RR. Genetic disruptions of O/E2 and O/E3 genes reveal involvement in olfactory receptor neuron projection. Development. 2004;131:1377–1388. doi: 10.1242/dev.01009. [DOI] [PubMed] [Google Scholar]
- Wang SS, Tsai RYL, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci. 1997;17:4149–4158. doi: 10.1523/JNEUROSCI.17-11-04149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier AR, Meir JY, Ross JM, Tavernarakis N, Driscoll M, Ishihara T, Katsura I, Miller DM., 3rd UNC-4/UNC-37-dependent repression of motor neuron-specific genes controls synaptic choice in Caenorhabditis elegans. Genes Dev. 1999;13:2774–2786. doi: 10.1101/gad.13.21.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol. 2005;25:4129–4137. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TT, McIntyre JC, Bose SC, Hardin D, Owen MC, McClintock TS. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005;483:251–262. doi: 10.1002/cne.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.