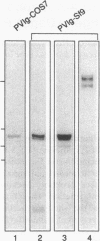
Abstract
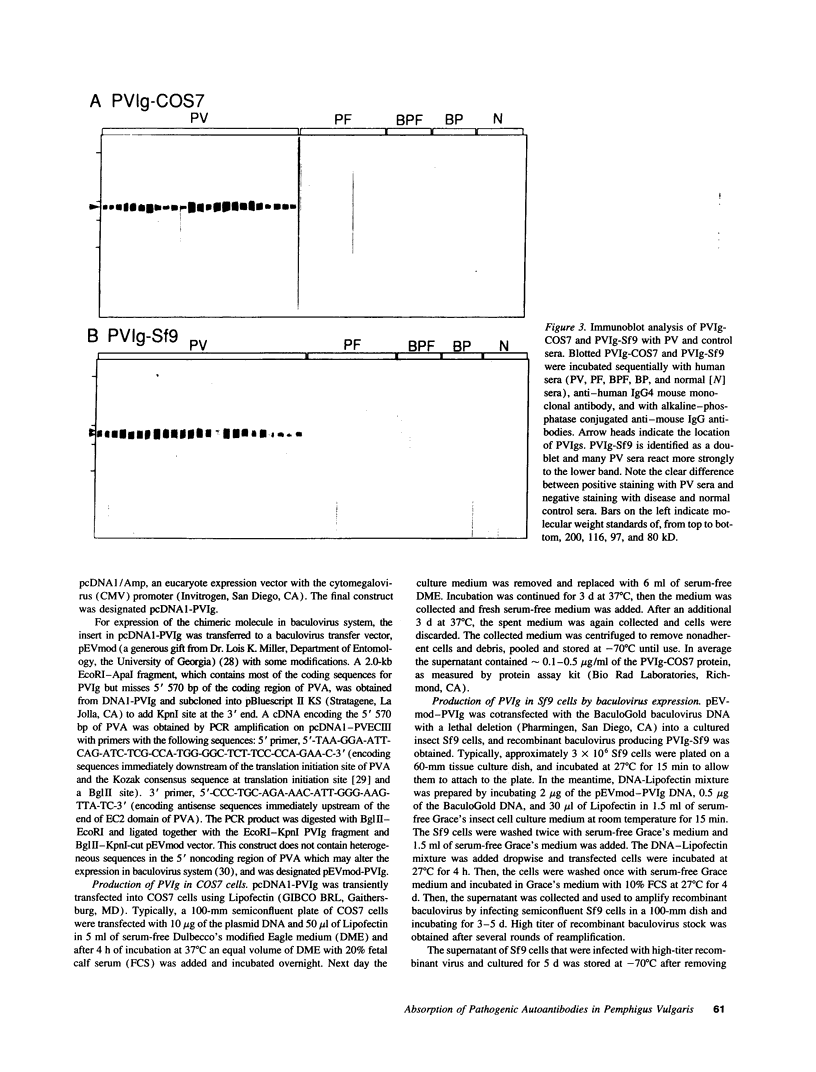
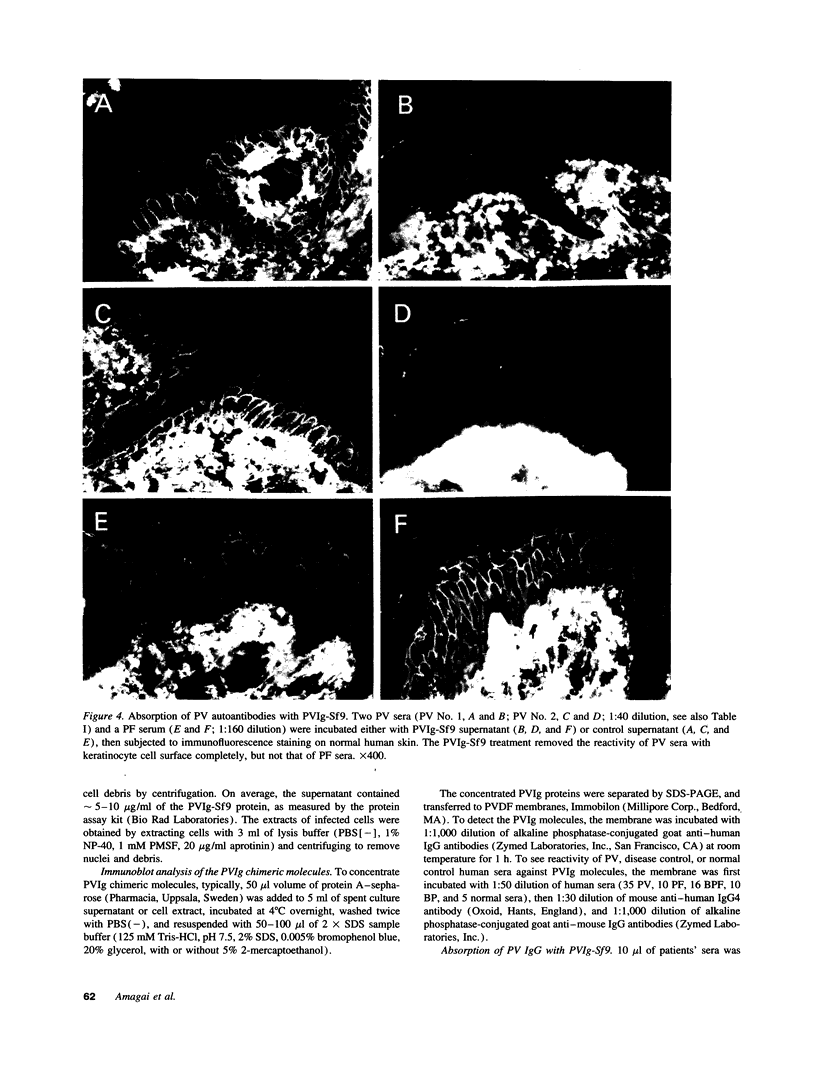
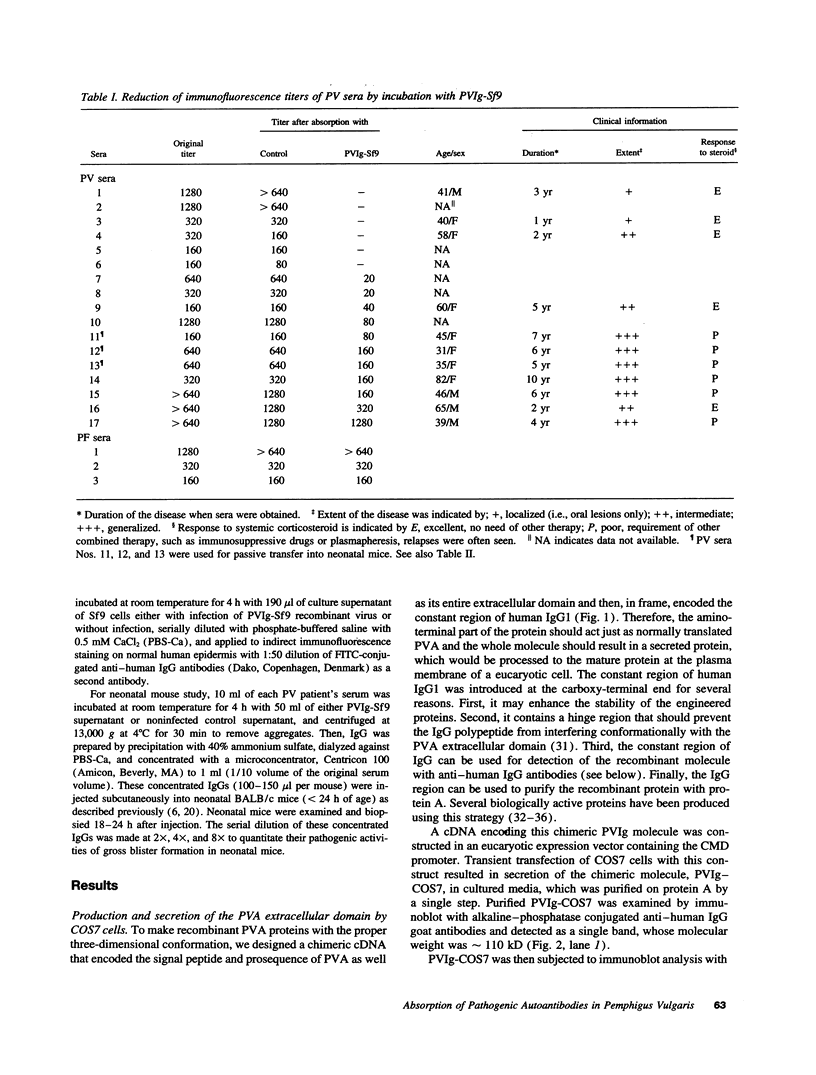
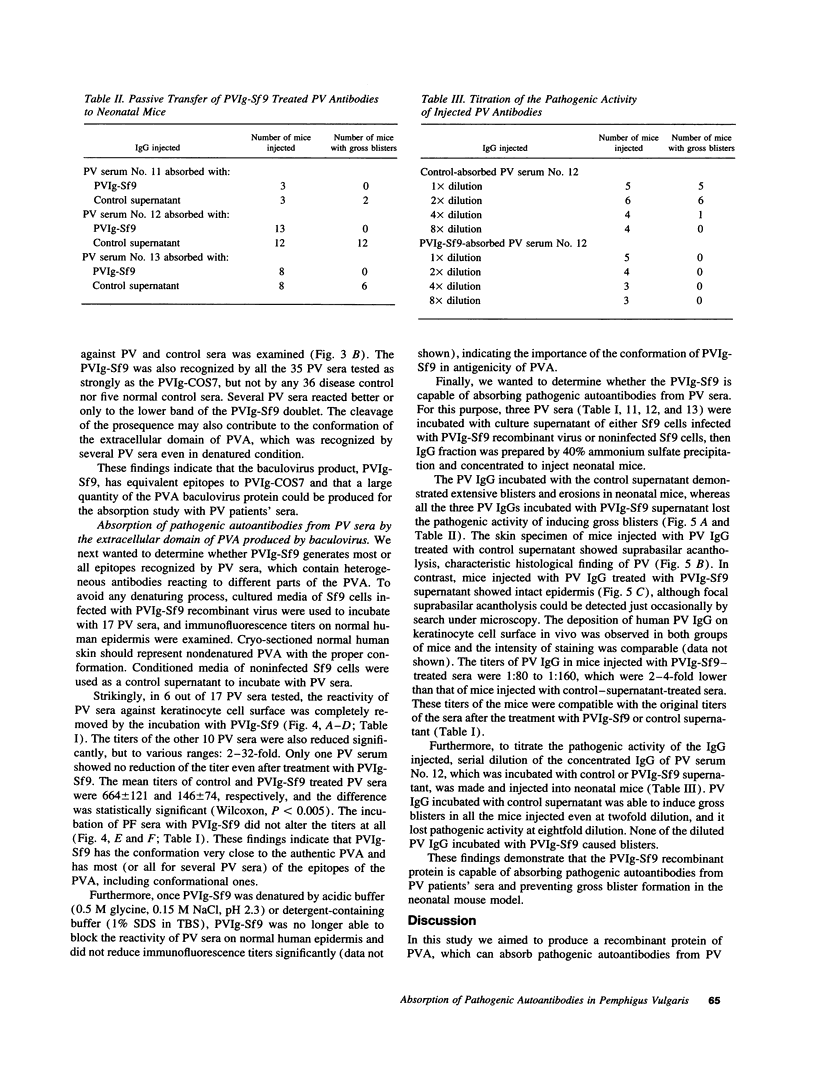
Pemphigus vulgaris (PV) is an autoimmune blistering disease, in which autoantibodies against PV antigen (PVA or Dsg3) play a pathogenic role in inducing blister formation. Bacterial fusion proteins of PVA failed to absorb pathogenic autoantibodies from PV patients' sera probably because they did not represent the proper conformation. Therefore, a chimeric protein, PVIg, consisting of the whole extracellular domain of PVA and the constant region of human IgG1, was produced in either in COS7 or in insect Sf9 eucaryotic cells. Both PVIg-COS7 and PVIg-Sf9 were recognized by all of the 35 PV sera tested, but not by any of 10 pemphigus foliaceus (PF), 16 Brazilian PF, 10 bullous pemphigoid, or five normal control sera. Incubation of PV patients' sera with PVIg-Sf9 removed heterogeneous autoantibodies and significantly reduced their immunofluorescence titers on normal human epidermis, although PVIg-Sf9 did not affect the titers of PF sera at all. Furthermore, PVIg-Sf9 absorbed pathogenic autoantibodies from patients' sera and prevented gross blister formation in a neonatal mouse model for pemphigus. These results indicate that this baculovirus product has the proper conformation of the authentic PVA and that its conformation is important in pathogenicity of pemphigus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Giudice G. J., Diaz L. A. Subclass reactivity of pemphigus foliaceus autoantibodies with recombinant human desmoglein. J Invest Dermatol. 1993 May;100(5):685–691. doi: 10.1111/1523-1747.ep12472348. [DOI] [PubMed] [Google Scholar]
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Amagai M., Karpati S., Prussick R., Klaus-Kovtun V., Stanley J. R. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J Clin Invest. 1992 Sep;90(3):919–926. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M., Klaus-Kovtun V., Stanley J. R. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991 Nov 29;67(5):869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- Amagai M., Kàrpàti S., Klaus-Kovtun V., Udey M. C., Stanley J. R. Extracellular domain of pemphigus vulgaris antigen (desmoglein 3) mediates weak homophilic adhesion. J Invest Dermatol. 1994 Apr;102(4):402–408. doi: 10.1111/1523-1747.ep12372164. [DOI] [PubMed] [Google Scholar]
- Anhalt G. J., Labib R. S., Voorhees J. J., Beals T. F., Diaz L. A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982 May 20;306(20):1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Kolanus W., Walz G., Fredman P., Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991 Oct 4;67(1):35–44. doi: 10.1016/0092-8674(91)90570-o. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Barkas T., Mauron A., Roth B., Alliod C., Tzartos S. J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987 Jan 2;235(4784):77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Eyre R. W., Stanley J. R. Identification of pemphigus vulgaris antigen extracted from normal human epidermis and comparison with pemphigus foliaceus antigen. J Clin Invest. 1988 Mar;81(3):807–812. doi: 10.1172/JCI113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin L., Hill J. E., Raynor K., Raszi L., Manabe M., Cowin P. Desmoglein shows extensive homology to the cadherin family of cell adhesion molecules. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1224–1230. doi: 10.1016/s0006-291x(05)80917-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Shafran K. M., Webber P. S., Lazarus G. S., Singer K. H. Anti-cell surface pemphigus autoantibody stimulates plasminogen activator activity of human epidermal cells. A mechanism for the loss of epidermal cohesion and blister formation. J Exp Med. 1983 Jan 1;157(1):259–272. doi: 10.1084/jem.157.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Ogawa M. M., Konohana A., Nishikawa T. Detection of pemphigus vulgaris and pemphigus foliaceus antigens by immunoblot analysis using different antigen sources. J Invest Dermatol. 1990 Mar;94(3):327–331. doi: 10.1111/1523-1747.ep12874456. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Yokoo K. M., Goldman R. D. Further analysis of pemphigus autoantibodies and their use in studies on the heterogeneity, structure, and function of desmosomes. J Cell Biol. 1986 Mar;102(3):1109–1117. doi: 10.1083/jcb.102.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. H., Geoghegan W. D., Jordon R. E. Pemphigus immunoglobulin G subclass autoantibodies: studies of reactivity with cultured human keratinocytes. J Lab Clin Med. 1990 Mar;115(3):324–331. [PubMed] [Google Scholar]
- Koch P. J., Walsh M. J., Schmelz M., Goldschmidt M. D., Zimbelmann R., Franke W. W. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990 Oct;53(1):1–12. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Veit M., Klenk H. D. Retarded processing of influenza virus hemagglutinin in insect cells. Virology. 1991 Jan;180(1):159–165. doi: 10.1016/0042-6822(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Levinovitz A., Mühlhoff J., Isenmann S., Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993 Apr;121(2):449–459. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlob P., Metzker A., Hazaz B., Rogovin H., Reisner S. H. Neonatal pemphigus vulgaris. Pediatrics. 1986 Dec;78(6):1102–1105. [PubMed] [Google Scholar]
- Miller J. E., Rico M. J., Hall R. P., 3rd IgG antibodies from patients with bullous pemphigoid bind to fusion proteins encoded by BPAg1 cDNA. J Invest Dermatol. 1993 Dec;101(6):779–782. doi: 10.1111/1523-1747.ep12371694. [DOI] [PubMed] [Google Scholar]
- Nilles L. A., Parry D. A., Powers E. E., Angst B. D., Wagner R. M., Green K. J. Structural analysis and expression of human desmoglein: a cadherin-like component of the desmosome. J Cell Sci. 1991 Aug;99(Pt 4):809–821. doi: 10.1242/jcs.99.4.809. [DOI] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Ogawa M. M., Hashimoto T., Nishikawa T., Castro R. M. IgG subclasses of intercellular antibodies in Brazilian pemphigus foliaceus--the relationship to complement fixing capability. Clin Exp Dermatol. 1989 Jan;14(1):29–31. doi: 10.1111/j.1365-2230.1989.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990 Oct;111(4):1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock B., Martins C. R., Theofilopoulos A. N., Balderas R. S., Anhalt G. J., Labib R. S., Futamura S., Rivitti E. A., Diaz L. A. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem). N Engl J Med. 1989 Jun 1;320(22):1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Jordon R. E. Correlation of pemphigoid and pemphigus antibody titres with activity of disease. Br J Dermatol. 1971 Jan;84(1):7–13. doi: 10.1111/j.1365-2133.1971.tb14190.x. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976 Aug;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- Shirakata Y., Shiraishi S., Sayama K., Miki Y. Subclass characteristics of IgG autoantibodies in bullous pemphigoid and pemphigus. J Dermatol. 1990 Nov;17(11):661–666. doi: 10.1111/j.1346-8138.1990.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Koulu L., Thivolet C. Distinction between epidermal antigens binding pemphigus vulgaris and pemphigus foliaceus autoantibodies. J Clin Invest. 1984 Aug;74(2):313–320. doi: 10.1172/JCI111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. R. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody-mediated diseases. J Clin Invest. 1989 May;83(5):1443–1448. doi: 10.1172/JCI114036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Hashimoto T., Amagai M., Shimizu N., Ikeguchi N., Tsubata T., Hasegawa A., Miki K., Nishikawa T. Characterization of bullous pemphigoid antibodies by use of recombinant bullous pemphigoid antigen proteins. J Invest Dermatol. 1991 Oct;97(4):725–728. doi: 10.1111/1523-1747.ep12484223. [DOI] [PubMed] [Google Scholar]
- Wang X. Z., Ooi B. G., Miller L. K. Baculovirus vectors for multiple gene expression and for occluded virus production. Gene. 1991 Apr;100:131–137. doi: 10.1016/0378-1119(91)90358-i. [DOI] [PubMed] [Google Scholar]
- Wheeler G. N., Parker A. E., Thomas C. L., Ataliotis P., Poynter D., Arnemann J., Rutman A. J., Pidsley S. C., Watt F. M., Rees D. A. Desmosomal glycoprotein DGI, a component of intercellular desmosome junctions, is related to the cadherin family of cell adhesion molecules. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4796–4800. doi: 10.1073/pnas.88.11.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Hashimoto T., Nishikawa T. IgG subclasses of intercellular and basement membrane zone antibodies: the relationship to the capability of complement fixation. J Invest Dermatol. 1989 Apr;92(4):585–587. doi: 10.1111/1523-1747.ep12709613. [DOI] [PubMed] [Google Scholar]