Abstract
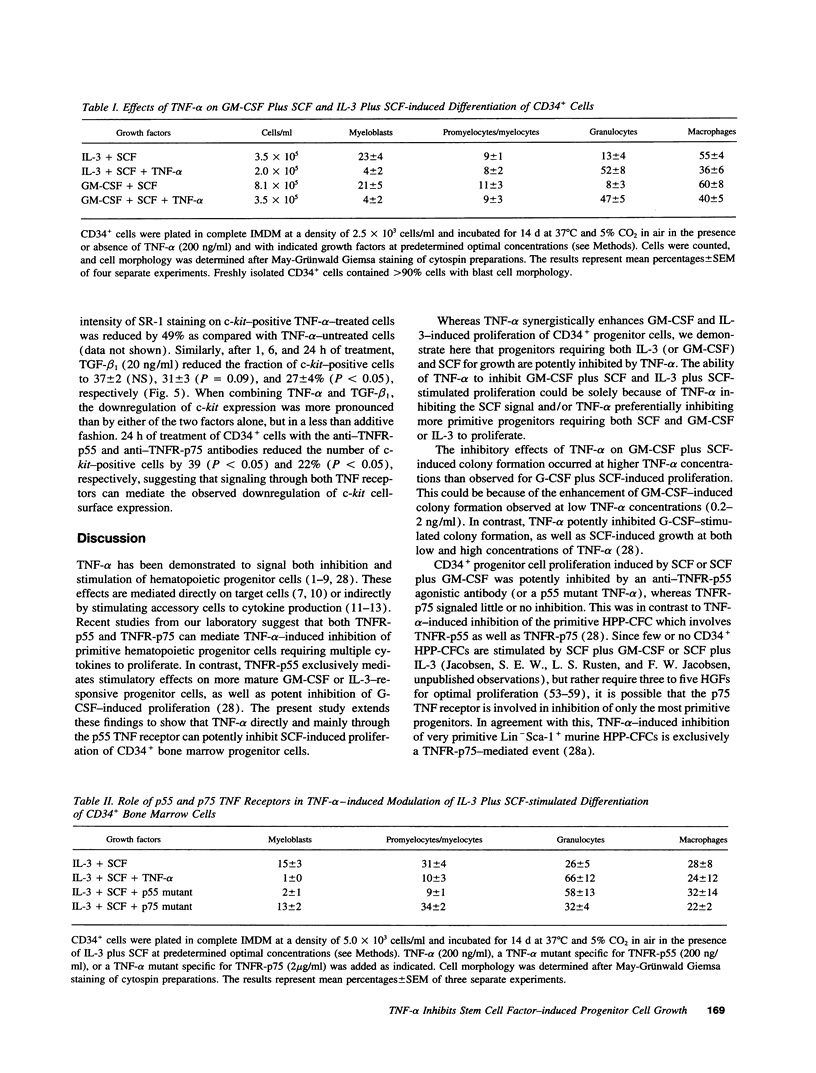
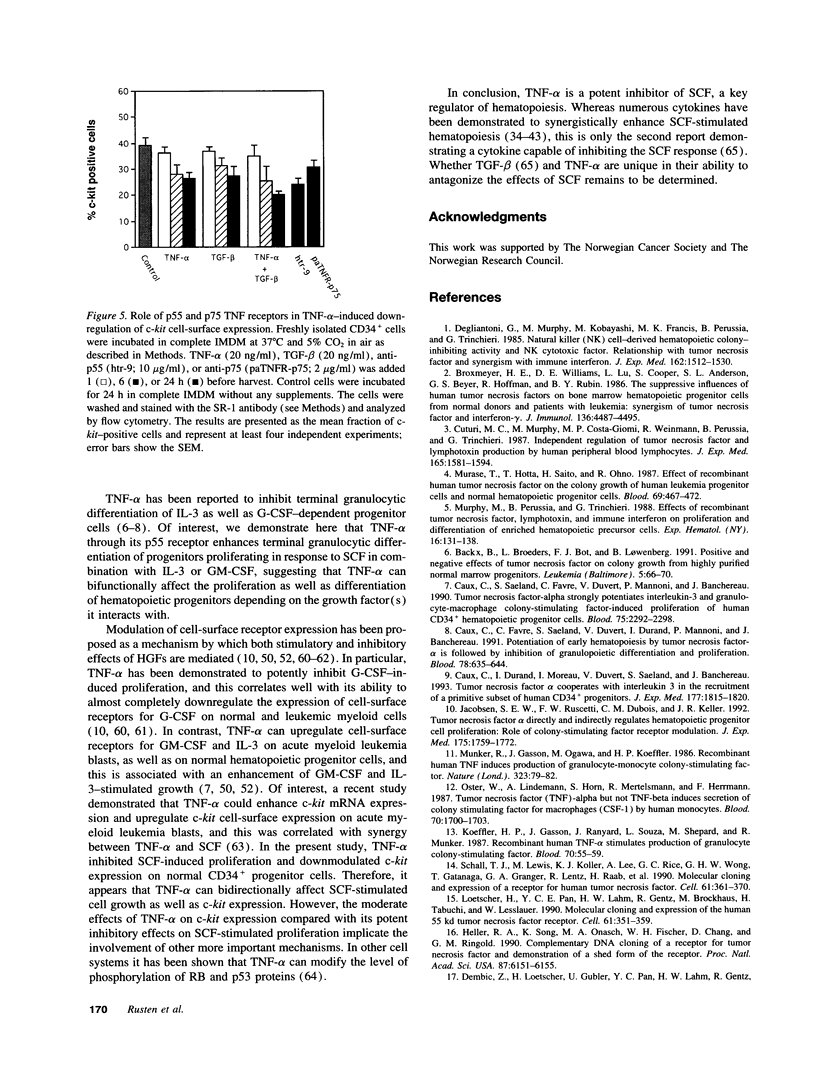
Stem cell factor (SCF), a key regulator of hematopoiesis, potently synergizes with a number of hematopoietic growth factors. However, little is known about growth factors capable of inhibiting the actions of SCF. TNF-alpha has been shown to act as a bidirectional regulator of myeloid cell proliferation and differentiation. This study was designed to examine interactions between TNF-alpha and SCF. Here, we demonstrate that TNF-alpha potently and directly inhibits SCF-stimulated proliferation of CD34+ hematopoietic progenitor cells. Furthermore, TNF-alpha blocked all colony formation stimulated by SCF in combination with granulocyte colony-stimulating factor (CSF) or CSF-1. The synergistic effect of SCF observed in combination with GM-CSF or IL-3 was also inhibited by TNF-alpha, resulting in colony numbers similar to those obtained in the absence of SCF. These effects of TNF-alpha were mediated through the p55 TNF receptor, whereas little or no inhibition was signaled through the p75 TNF receptor. Finally, TNF-alpha downregulated c-kit cell-surface expression on CD34+ bone marrow cells, and this was predominantly a p55 TNF receptor-mediated event as well.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Vannier E., Cowley S., Jiang S. X., Chi S., Dinarello C. A., Zsebo K. M., Groopman J. E. Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood. 1992 Jan 15;79(2):365–371. [PubMed] [Google Scholar]
- Backx B., Broeders L., Bot F. J., Löwenberg B. Positive and negative effects of tumor necrosis factor on colony growth from highly purified normal marrow progenitors. Leukemia. 1991 Jan;5(1):66–70. [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor. Improving on the formula. Nature. 1993 Jan 21;361(6409):206–207. doi: 10.1038/361206a0. [DOI] [PubMed] [Google Scholar]
- Bernstein I. D., Andrews R. G., Zsebo K. M. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+lin- cells, and the generation of colony-forming cell progeny from CD34+lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991 Jun 1;77(11):2316–2321. [PubMed] [Google Scholar]
- Brach M. A., Bühring H. J., Gruss H. J., Ashman L. K., Ludwig W. D., Mertelsmann R. H., Herrmann F. Functional expression of c-kit by acute myelogenous leukemia blasts is enhanced by tumor necrosis factor-alpha through posttranscriptional mRNA stabilization by a labile protein. Blood. 1992 Sep 1;80(5):1224–1230. [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979 Dec;54(6):1446–1450. [PubMed] [Google Scholar]
- Brandt J., Briddell R. A., Srour E. F., Leemhuis T. B., Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992 Feb 1;79(3):634–641. [PubMed] [Google Scholar]
- Briddell R. A., Bruno E., Cooper R. J., Brandt J. E., Hoffman R. Effect of c-kit ligand on in vitro human megakaryocytopoiesis. Blood. 1991 Dec 1;78(11):2854–2859. [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Lin N., Zsebo K. M., Birkett N. C., Smith K. A., Bernstein I. D., Papayannopoulou T. Isolation and characterization of a monoclonal antibody that recognizes the human c-kit receptor. Blood. 1992 Jan 15;79(2):338–346. [PubMed] [Google Scholar]
- Broxmeyer H. E., Cooper S., Lu L., Hangoc G., Anderson D., Cosman D., Lyman S. D., Williams D. E. Effect of murine mast cell growth factor (c-kit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood. 1991 May 15;77(10):2142–2149. [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Caux C., Durand I., Moreau I., Duvert V., Saeland S., Banchereau J. Tumor necrosis factor alpha cooperates with interleukin 3 in the recruitment of a primitive subset of human CD34+ progenitors. J Exp Med. 1993 Jun 1;177(6):1815–1820. doi: 10.1084/jem.177.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Favre C., Saeland S., Duvert V., Durand I., Mannoni P., Banchereau J. Potentiation of early hematopoiesis by tumor necrosis factor-alpha is followed by inhibition of granulopoietic differentiation and proliferation. Blood. 1991 Aug 1;78(3):635–644. [PubMed] [Google Scholar]
- Caux C., Saeland S., Favre C., Duvert V., Mannoni P., Banchereau J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1990 Jun 15;75(12):2292–2298. [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembic Z., Loetscher H., Gubler U., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Lesslauer W. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine. 1990 Jul;2(4):231–237. doi: 10.1016/1043-4666(90)90022-l. [DOI] [PubMed] [Google Scholar]
- Elbaz O., Budel L. M., Hoogerbrugge H., Touw I. P., Delwel R., Mahmoud L. A., Löwenberg B. Tumor necrosis factor downregulates granulocyte-colony-stimulating factor receptor expression on human acute myeloid leukemia cells and granulocytes. J Clin Invest. 1991 Mar;87(3):838–841. doi: 10.1172/JCI115087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz O., Budel L. M., Hoogerbrugge H., Touw I. P., Delwel R., Mahmoud L. A., Löwenberg B. Tumor necrosis factor regulates the expression of granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors on human acute myeloid leukemia cells. Blood. 1991 Mar 1;77(5):989–995. [PubMed] [Google Scholar]
- Everaerdt B., Brouckaert P., Shaw A., Fiers W. Four different interleukin-1 species sensitize to the lethal action of tumour necrosis factor. Biochem Biophys Res Commun. 1989 Aug 30;163(1):378–385. doi: 10.1016/0006-291x(89)92146-3. [DOI] [PubMed] [Google Scholar]
- Fransen L., Ruysschaert M. R., Van der Heyden J., Fiers W. Recombinant tumor necrosis factor: species specificity for a variety of human and murine transformed cell lines. Cell Immunol. 1986 Jun;100(1):260–267. doi: 10.1016/0008-8749(86)90025-0. [DOI] [PubMed] [Google Scholar]
- Gehr G., Gentz R., Brockhaus M., Loetscher H., Lesslauer W. Both tumor necrosis factor receptor types mediate proliferative signals in human mononuclear cell activation. J Immunol. 1992 Aug 1;149(3):911–917. [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Cao X., Chua S. P., Tan Y. H. Okadaic acid mimics multiple changes in early protein phosphorylation and gene expression induced by tumor necrosis factor or interleukin-1. J Biol Chem. 1992 Jan 25;267(3):1846–1852. [PubMed] [Google Scholar]
- Haylock D. N., To L. B., Dowse T. L., Juttner C. A., Simmons P. J. Ex vivo expansion and maturation of peripheral blood CD34+ cells into the myeloid lineage. Blood. 1992 Sep 15;80(6):1405–1412. [PubMed] [Google Scholar]
- Heimfeld S., Hudak S., Weissman I., Rennick D. The in vitro response of phenotypically defined mouse stem cells and myeloerythroid progenitors to single or multiple growth factors. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9902–9906. doi: 10.1073/pnas.88.21.9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R. A., Song K., Onasch M. A., Fischer W. H., Chang D., Ringold G. M. Complementary DNA cloning of a receptor for tumor necrosis factor and demonstration of a shed form of the receptor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6151–6155. doi: 10.1073/pnas.87.16.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Nicholls S., Zsebo K., Dexter T. M. Stem cell factor directly stimulates the development of enriched granulocyte-macrophage colony-forming cells and promotes the effects of other colony-stimulating factors. Blood. 1992 Nov 1;80(9):2230–2236. [PubMed] [Google Scholar]
- Ikebuchi K., Clark S. C., Ihle J. N., Souza L. M., Ogawa M. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1988 May;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Shaw A. R., Keller G. Net increase of pluripotential hematopoietic precursors in suspension culture in response to IL-1 and IL-3. J Immunol. 1989 Apr 1;142(7):2332–2337. [PubMed] [Google Scholar]
- Jacobsen S. E., Ruscetti F. W., Dubois C. M., Keller J. R. Tumor necrosis factor alpha directly and indirectly regulates hematopoietic progenitor cell proliferation: role of colony-stimulating factor receptor modulation. J Exp Med. 1992 Jun 1;175(6):1759–1772. doi: 10.1084/jem.175.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen S. E., Ruscetti F. W., Dubois C. M., Wine J., Keller J. R. Induction of colony-stimulating factor receptor expression on hematopoietic progenitor cells: proposed mechanism for growth factor synergism. Blood. 1992 Aug 1;80(3):678–687. [PubMed] [Google Scholar]
- Jacobsen S. E., Veiby O. P., Smeland E. B. Cytotoxic lymphocyte maturation factor (interleukin 12) is a synergistic growth factor for hematopoietic stem cells. J Exp Med. 1993 Aug 1;178(2):413–418. doi: 10.1084/jem.178.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Ranyard J., Souza L., Shepard M., Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987 Jul;70(1):55–59. [PubMed] [Google Scholar]
- Lewis M., Tartaglia L. A., Lee A., Bennett G. L., Rice G. C., Wong G. H., Chen E. Y., Goeddel D. V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard D., Ewalenko P., Delmotte J. J., Renard N., Lejeune F. J. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992 Jan;10(1):52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Stueber D., Banner D., Mackay F., Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993 Dec 15;268(35):26350–26357. [PubMed] [Google Scholar]
- Martin F. H., Suggs S. V., Langley K. E., Lu H. S., Ting J., Okino K. H., Morris C. F., McNiece I. K., Jacobsen F. W., Mendiaz E. A. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990 Oct 5;63(1):203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- McNiece I. K., Bertoncello I., Keller J. R., Ruscetti F. W., Hartley C. A., Zsebo K. M. Transforming growth factor beta inhibits the action of stem cell factor on mouse and human hematopoietic progenitors. Int J Cell Cloning. 1992 Mar;10(2):80–86. doi: 10.1002/stem.5530100204. [DOI] [PubMed] [Google Scholar]
- McNiece I. K., Langley K. E., Zsebo K. M. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991 Mar;19(3):226–231. [PubMed] [Google Scholar]
- McNiece I. K., Stewart F. M., Deacon D. M., Temeles D. S., Zsebo K. M., Clark S. C., Quesenberry P. J. Detection of a human CFC with a high proliferative potential. Blood. 1989 Aug 1;74(2):609–612. [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- Muench M. O., Firpo M. T., Moore M. A. Bone marrow transplantation with interleukin-1 plus kit-ligand ex vivo expanded bone marrow accelerates hematopoietic reconstitution in mice without the loss of stem cell lineage and proliferative potential. Blood. 1993 Jun 15;81(12):3463–3473. [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Murase T., Hotta T., Saito H., Ohno R. Effect of recombinant human tumor necrosis factor on the colony growth of human leukemia progenitor cells and normal hematopoietic progenitor cells. Blood. 1987 Feb;69(2):467–472. [PubMed] [Google Scholar]
- Murphy M., Perussia B., Trinchieri G. Effects of recombinant tumor necrosis factor, lymphotoxin, and immune interferon on proliferation and differentiation of enriched hematopoietic precursor cells. Exp Hematol. 1988 Feb;16(2):131–138. [PubMed] [Google Scholar]
- Oster W., Lindemann A., Horn S., Mertelsmann R., Herrmann F. Tumor necrosis factor (TNF)-alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood. 1987 Nov;70(5):1700–1703. [PubMed] [Google Scholar]
- Retsas S., Leslie M., Bottomley D. Intralesional tumour necrosis factor combined with interferon gamma in metastatic melanoma. BMJ. 1989 May 13;298(6683):1290–1291. doi: 10.1136/bmj.298.6683.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Caux C., Kitamura T., Watanabe Y., Arai K., Banchereau J., Miyajima A. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood. 1993 Aug 1;82(3):752–761. [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Shieh J. H., Peterson R. H., Moore M. A. Modulation of granulocyte colony-stimulating factor receptors on murine peritoneal exudate macrophages by tumor necrosis factor-alpha. J Immunol. 1991 Apr 15;146(8):2648–2653. [PubMed] [Google Scholar]
- Smeland E. B., Funderud S., Kvalheim G., Gaudernack G., Rasmussen A. M., Rusten L., Wang M. Y., Tindle R. W., Blomhoff H. K., Egeland T. Isolation and characterization of human hematopoietic progenitor cells: an effective method for positive selection of CD34+ cells. Leukemia. 1992 Aug;6(8):845–852. [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Lyman S. D., Sudo T., Clark S. C., Ogawa M. Enhancement of murine hematopoiesis by synergistic interactions between steel factor (ligand for c-kit), interleukin-11, and other early acting factors in culture. Blood. 1992 Jun 1;79(11):2855–2860. [PubMed] [Google Scholar]
- Van Ostade X., Vandenabeele P., Everaerdt B., Loetscher H., Gentz R., Brockhaus M., Lesslauer W., Tavernier J., Brouckaert P., Fiers W. Human TNF mutants with selective activity on the p55 receptor. Nature. 1993 Jan 21;361(6409):266–269. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kitamura T., Hayashida K., Miyajima A. Monoclonal antibody against the common beta subunit (beta c) of the human interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor receptors shows upregulation of beta c by IL-1 and tumor necrosis factor-alpha. Blood. 1992 Nov 1;80(9):2215–2220. [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Wypych J., McNiece I. K., Lu H. S., Smith K. A., Karkare S. B., Sachdev R. K., Yuschenkoff V. N., Birkett N. C., Williams L. R. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990 Oct 5;63(1):195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]