Abstract
To gain insight into the mechanisms of autoantibody induction, sera from 40 patients with systemic lupus erythematosus (SLE) were tested by ELISAs for antibody binding to denatured individual histones, native histone-histone complexes, histone-DNA subnucleosome complexes, three forms of chromatin, and DNA. Whole chromatin was the most reactive substrate, with 88% of the patients positive. By chi-square analysis, only the presence of anti-(H2A-H2B), anti-[(H2A-H2B)-DNA], and antichromatin were correlated with kidney disease measured by proteinuria > 0.5 g/d. SLE patients could be divided into two groups based on their antibody-binding pattern to the above substrates. Antibodies from about half of the patients reacted with chromatin and the (H2A-H2B)-DNA subnucleosome complex but displayed very low or no reactivity with native DNA or the (H3-H4)2-DNA subnucleosome complex. An additional third of the patients had antibody reactivity to chromatin, as well as to both subnucleosome structures and DNA. Strikingly, all sera that bound to any of the components of chromatin also bound to whole chromatin, and adsorption with chromatin removed 85-100% of reactivity to (H2A-H2B)-DNA, (H3-H4)2-DNA, and native DNA. Individual sera often bound to several different epitopes on chromatin, with some epitopes requiring quaternary protein-DNA interactions. These results are consistent with chromatin being a potent immunogenic stimulus in SLE. Taken together with previous studies, we suggest that antibody activity to the (H2A-H2B)-DNA component signals the initial breakdown of immune tolerance whereas responses to (H3-H4)2-DNA and native DNA reflect subsequent global loss of tolerance to chromatin.
Full text
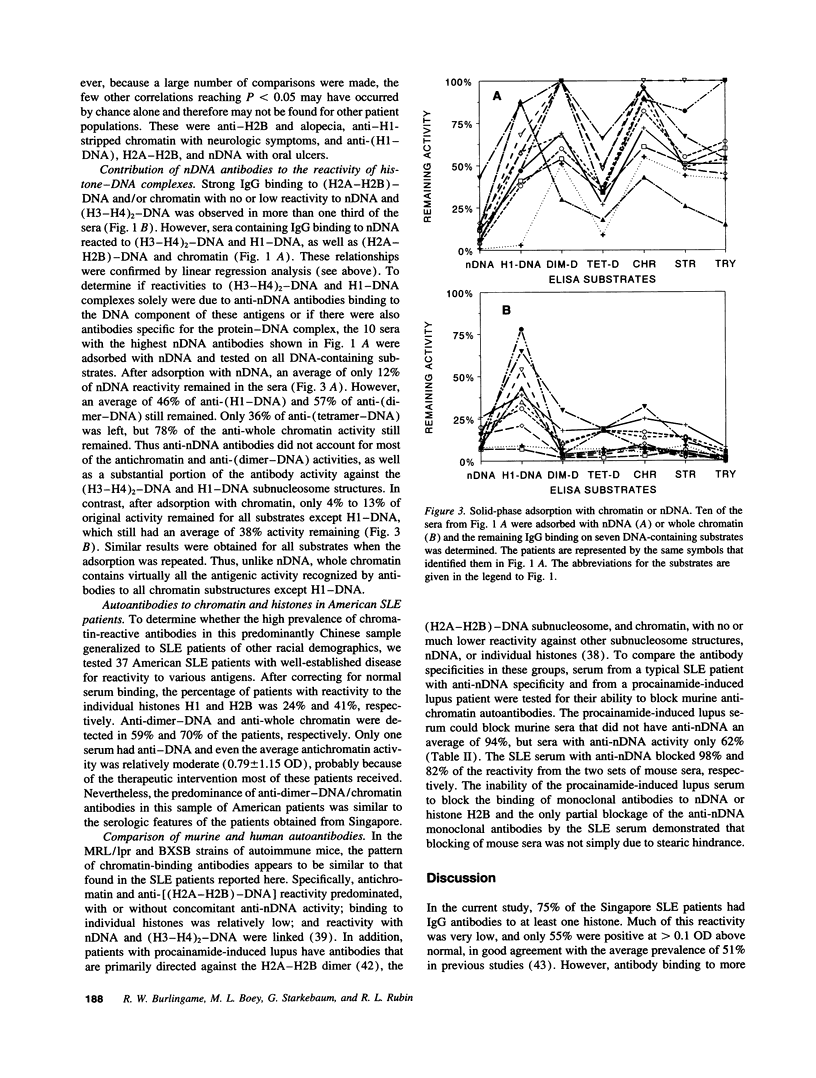
PDF




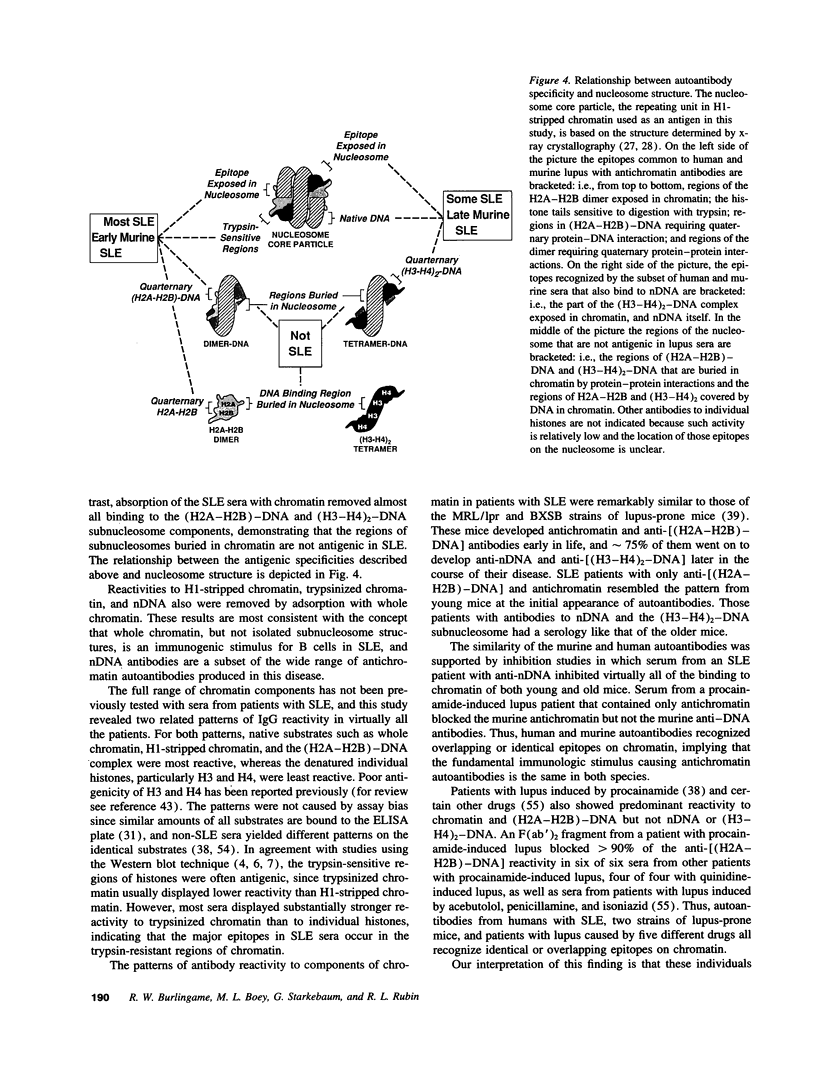


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arana R., Seligmann M. Antibodies to native and denatured deoxyribonucleic acid in systemic lupus erythematosus. J Clin Invest. 1967 Nov;46(11):1867–1882. doi: 10.1172/JCI105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arents G., Burlingame R. W., Wang B. C., Love W. E., Moudrianakis E. N. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Hamilton R. G., Roebber M. G., Harley J. B., Reichlin M. Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol. 1988 Dec;15(12):1773–1776. [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Boey M. L., Peebles C. L., Tsay G., Feng P. H., Tan E. M. Clinical and autoantibody correlations in Orientals with systemic lupus erythematosus. Ann Rheum Dis. 1988 Nov;47(11):918–923. doi: 10.1136/ard.47.11.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Anti-histone autoantibodies recognize centromeric heterochromatin in metaphase chromosomes and hidden epitopes in interphase cells. Hum Antibodies Hybridomas. 1992 Jan;3(1):40–47. [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L., Balderas R. S., Theofilopoulos A. N. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J Clin Invest. 1993 Apr;91(4):1687–1696. doi: 10.1172/JCI116378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest. 1991 Aug;88(2):680–690. doi: 10.1172/JCI115353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J Immunol Methods. 1990 Dec 5;134(2):187–199. doi: 10.1016/0022-1759(90)90380-e. [DOI] [PubMed] [Google Scholar]
- Cohen M. G., Pollard K. M., Webb J. Antibodies to histones in systemic lupus erythematosus: prevalence, specificity, and relationship to clinical and laboratory features. Ann Rheum Dis. 1992 Jan;51(1):61–66. doi: 10.1136/ard.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Tan E. M. Antibodies to histones in drug-induced and idiopathic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):560–567. doi: 10.1172/JCI109161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman D. D., Urowitz M. B., Keystone E. C. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serologic features. Am J Med. 1979 Feb;66(2):210–215. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Baxevanis A. D., Moudrianakis E. N. Spectropolarimetric analysis of the core histone octamer and its subunits. Biochemistry. 1990 Jan 30;29(4):965–972. doi: 10.1021/bi00456a018. [DOI] [PubMed] [Google Scholar]
- Gohill J., Cary P. D., Couppez M., Fritzler M. J. Antibodies from patients with drug-induced and idiopathic lupus erythematosus react with epitopes restricted to the amino and carboxyl termini of histone. J Immunol. 1985 Nov;135(5):3116–3121. [PubMed] [Google Scholar]
- Gompertz N. R., Isenberg D. A., Turner B. M. Correlation between clinical features of systemic lupus erythematosus and levels of antihistone antibodies of the IgG, IgA, and IgM isotypes. Ann Rheum Dis. 1990 Jul;49(7):524–527. doi: 10.1136/ard.49.7.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN H., DEICHER H. R. The reaction of the lupus erythematosus (L.E.) cell factor with deoxyribonucleoprotein of the cell nucleus. J Clin Invest. 1959 Nov;38:2059–2072. doi: 10.1172/JCI103984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., van Venrooij W. J. Epitope patterns of anti-RNP antibodies in rheumatic diseases. Evidence for an antigen-driven autoimmune response. Arthritis Rheum. 1990 Jun;33(6):834–841. doi: 10.1002/art.1780330610. [DOI] [PubMed] [Google Scholar]
- Isenberg D. A., Shoenfeld Y., Schwartz R. S. Multiple serologic reactions and their relationship to clinical activity in systemic lupus erythematosus. Arthritis Rheum. 1984 Feb;27(2):132–138. doi: 10.1002/art.1780270203. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Lafferty J. A., Portanova J. P., Rubin R. L., Tan E. M. Monoclonal anti-histone autoantibodies derived from murine models of lupus. J Immunol. 1984 Nov;133(5):2554–2559. [PubMed] [Google Scholar]
- Krippner H., Springer B., Merle S., Pirlet K. Antibodies to histones of the IgG and IgM class in systemic lupus erythematosus. Clin Exp Immunol. 1984 Oct;58(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- Ludivico C. L., Zweiman B., Myers A. R., Hebert J., Green P. A. Predictive value of anti-DNA antibody and selected laboratory studies in systemic lupus erythematosus. J Rheumatol. 1980 Nov-Dec;7(6):843–849. [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Madaio M. P., Hodder S., Schwartz R. S., Stollar B. D. Responsiveness of autoimmune and normal mice to nucleic acid antigens. J Immunol. 1984 Feb;132(2):872–876. [PubMed] [Google Scholar]
- Marion T. N., Tillman D. M., Jou N. T. Interclonal and intraclonal diversity among anti-DNA antibodies from an (NZB x NZW)F1 mouse. J Immunol. 1990 Oct 1;145(7):2322–2332. [PubMed] [Google Scholar]
- Mencke A. J., Rill R. L. Circular dichroism and thermal denaturation studies of subnucleosomes and their relationships to nucleosome structure. Biochemistry. 1982 Aug 31;21(18):4362–4370. doi: 10.1021/bi00261a027. [DOI] [PubMed] [Google Scholar]
- Mohan C., Adams S., Stanik V., Datta S. K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993 May 1;177(5):1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M., Kanayama Y., Amastu K., Negoro N., Kohda S., Takeda T., Inoue T. Significance of enzyme linked immunosorbent assay (ELISA) for antibodies to double stranded and single stranded DNA in patients with lupus nephritis: correlation with severity of renal histology. Ann Rheum Dis. 1993 Jan;52(1):14–20. doi: 10.1136/ard.52.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Oohara I., Wada A. Spectroscopic studies on histone-DNA interactions. I. The interaction of histone (H2A, H2B) dimer with DNA: DNA sequence dependence. J Mol Biol. 1987 Jul 20;196(2):389–397. doi: 10.1016/0022-2836(87)90699-1. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Grudier J. P., Gilkeson G. S. A role for immunogenic DNA in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1990 Feb;33(2):153–159. doi: 10.1002/art.1780330202. [DOI] [PubMed] [Google Scholar]
- Portanova J. P., Arndt R. E., Tan E. M., Kotzin B. L. Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J Immunol. 1987 Jan 15;138(2):446–451. [PubMed] [Google Scholar]
- Reichlin M., Reichlin M. W. Autoantibodies to the Ro/SS-A particle react preferentially with the human antigen. J Autoimmun. 1989 Aug;2(4):359–365. doi: 10.1016/0896-8411(89)90164-9. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Lupus erythematosus (LE) factors recognize both nucleosomes and viable human leucocytes. Scand J Immunol. 1981;13(6):597–604. doi: 10.1111/j.1365-3083.1981.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Robitaille P., Tan E. M. Relationship between deoxyribonucleoprotein and deoxyribonucleic acid antibodies in systemic lupus erythematosus. J Clin Invest. 1973 Feb;52(2):316–323. doi: 10.1172/JCI107187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Bell S. A., Burlingame R. W. Autoantibodies associated with lupus induced by diverse drugs target a similar epitope in the (H2A-H2B)-DNA complex. J Clin Invest. 1992 Jul;90(1):165–173. doi: 10.1172/JCI115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Tsay G., Pollard K. M. Pseudoautoimmunity in normal mice: anti-histone antibodies elicited by immunization versus induction during graft-versus-host reaction. Clin Immunol Immunopathol. 1990 Feb;54(2):320–332. doi: 10.1016/0090-1229(90)90093-6. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Theofilopoulos A. N. Monoclonal autoantibodies reacting with multiple structurally related and unrelated macromolecules. Int Rev Immunol. 1988 Mar;3(1-2):71–95. doi: 10.3109/08830188809051183. [DOI] [PubMed] [Google Scholar]
- Schmiedeke T. M., Stöckl F. W., Weber R., Sugisaki Y., Batsford S. R., Vogt A. Histones have high affinity for the glomerular basement membrane. Relevance for immune complex formation in lupus nephritis. J Exp Med. 1989 Jun 1;169(6):1879–1894. doi: 10.1084/jem.169.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Reactions of systemic lupus erythematosus sera with histone fractions and histone-DNA complexes. Arthritis Rheum. 1971 Jul-Aug;14(4):485–492. doi: 10.1002/art.1780140408. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Natali P. G. Comparative study of antibodies to native and denatured DNA. J Immunol. 1970 Apr;104(4):902–906. [PubMed] [Google Scholar]
- Thomas J. O., Wilson C. M., Hardin J. A. The major core histone antigenic determinants in systemic lupus erythematosus are in the trypsin-sensitive regions. FEBS Lett. 1984 Apr 9;169(1):90–96. doi: 10.1016/0014-5793(84)80295-1. [DOI] [PubMed] [Google Scholar]
- Totoritis M. C., Tan E. M., McNally E. M., Rubin R. L. Association of antibody to histone complex H2A-H2B with symptomatic procainamide-induced lupus. N Engl J Med. 1988 Jun 2;318(22):1431–1436. doi: 10.1056/NEJM198806023182204. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E., Limburg P. C., Kallenberg C. G. Rises in anti-double stranded DNA antibody levels prior to exacerbations of systemic lupus erythematosus are not merely due to polyclonal B cell activation. Clin Immunol Immunopathol. 1991 Apr;59(1):117–128. doi: 10.1016/0090-1229(91)90086-p. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]



