Abstract
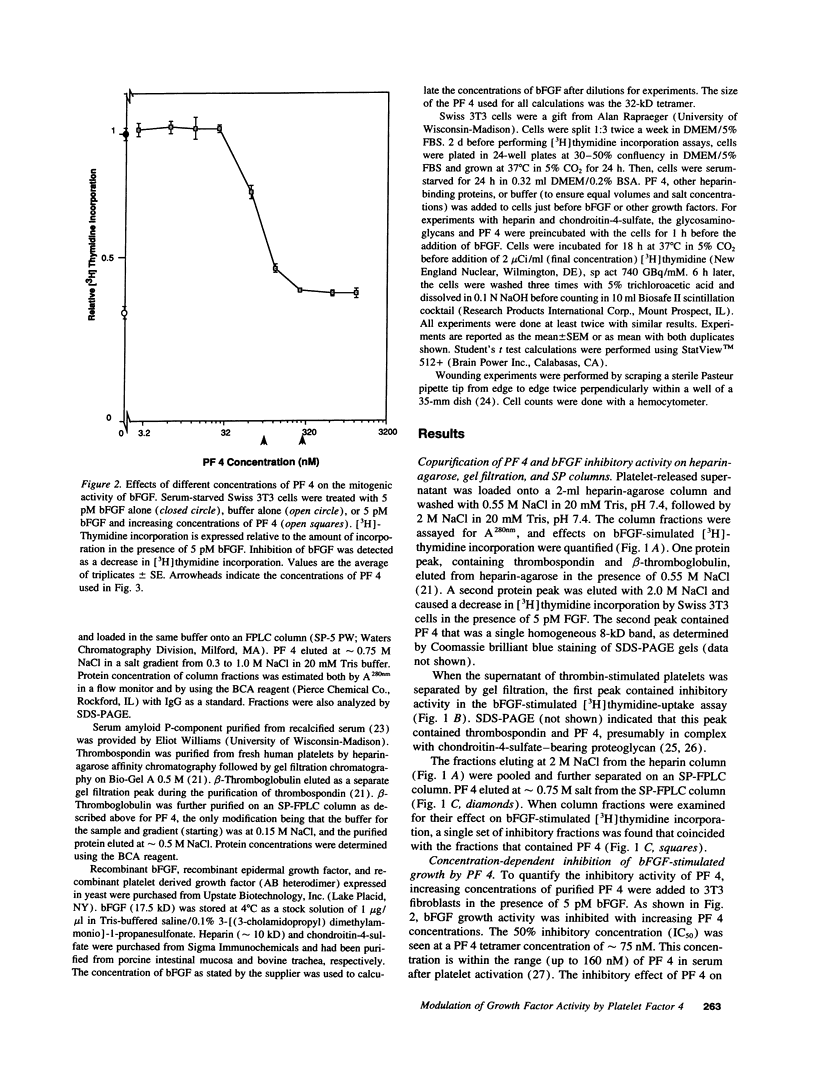
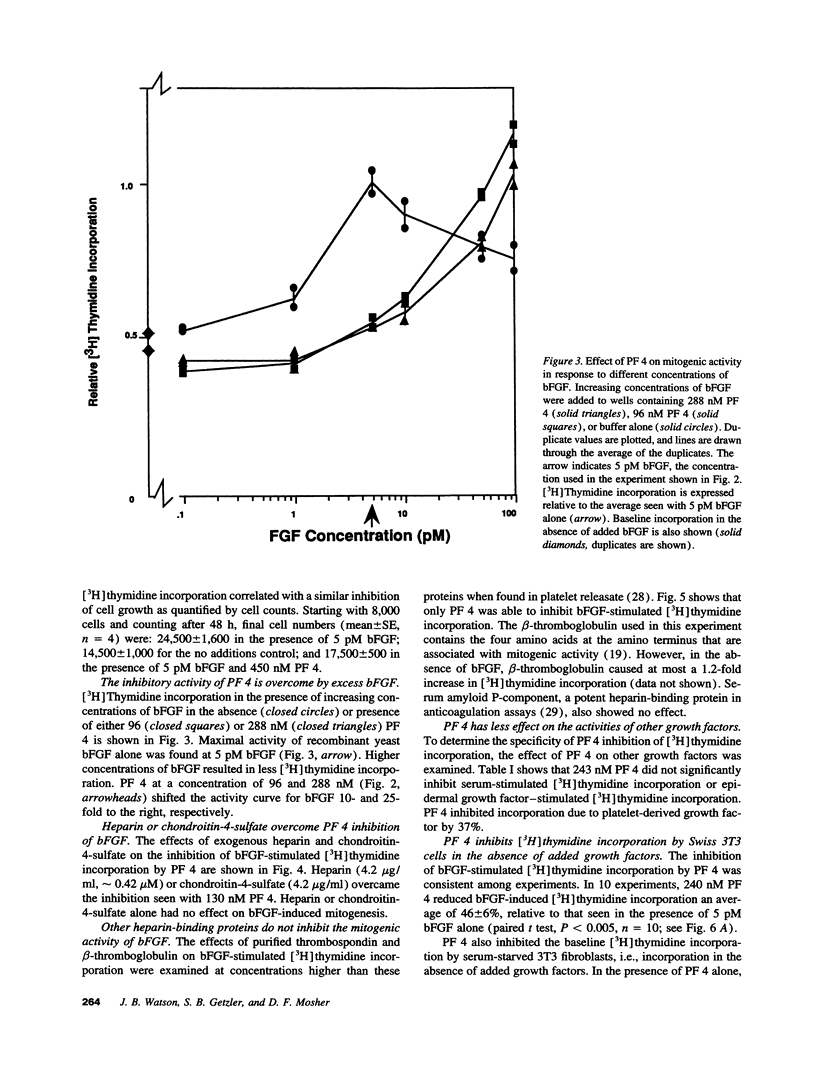
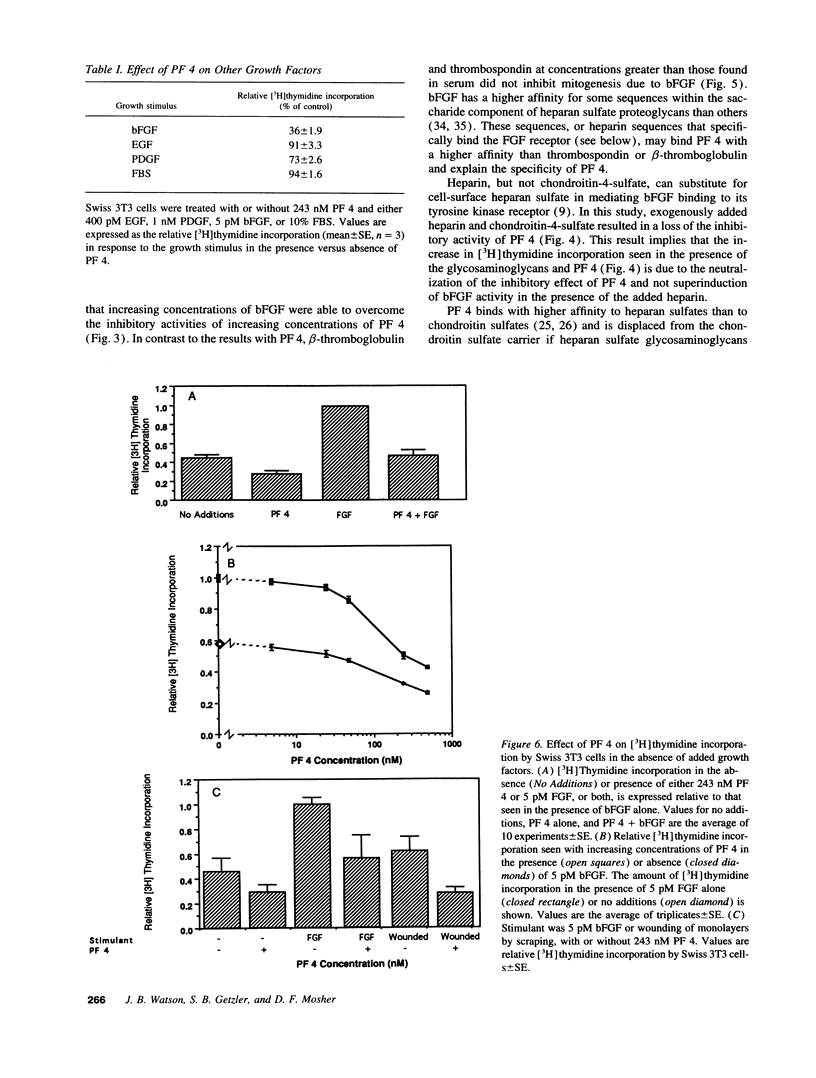
Basic fibroblast growth factor (bFGF) has been shown to stimulate cell proliferation after vascular injury. The mitogenic activity of bFGF requires interactions with both a high affinity receptor and a cell-surface heparan sulfate proteoglycan. We tested the ability of platelet factor 4 (PF 4) and other platelet heparin-binding proteins to modulate bFGF-stimulated [3H]thymidine incorporation into fibroblasts. The supernatant of thrombin-stimulated platelets contained an inhibitor of bFGF-induced mitogenesis; this activity coeluted with PF 4 upon gel filtration, heparin-agarose, and ion-exchange chromatography. Purified thrombospondin and beta-thromboglobulin did not inhibit the mitogenic activity of bFGF. PF 4 inhibited the activity of 5 pM bFGF with 50% inhibitory concentration of 75 nM. Purified PF 4 also inhibited the basal incorporation of [3H]thymidine into 3T3 fibroblasts and the increased [3H]thymidine incorporation occurring after wounding of a cell monolayer. PF 4 did not affect the mitogenic activity of serum. Inhibition of bFGF activity by PF 4 could be overcome by exogenous heparin or chondroitin-4-sulfate, suggesting that inhibition of mitogenesis is caused by binding of PF 4 to cell-surface glycosaminoglycans. These results indicate that an important role of PF 4 released at sites of vascular injury and platelet activation is to control cellular proliferation caused by the release of bFGF from ruptured cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuevas P., Gonzalez A. M., Carceller F., Baird A. Vascular response to basic fibroblast growth factor when infused onto the normal adventitia or into the injured media of the rat carotid artery. Circ Res. 1991 Aug;69(2):360–369. doi: 10.1161/01.res.69.2.360. [DOI] [PubMed] [Google Scholar]
- Damon D. H., D'Amore P. A., Wagner J. A. Sulfated glycosaminoglycans modify growth factor-induced neurite outgrowth in PC12 cells. J Cell Physiol. 1988 May;135(2):293–300. doi: 10.1002/jcp.1041350217. [DOI] [PubMed] [Google Scholar]
- DeFeudis F. V. Coronary atherosclerosis: current therapeutic approaches and future trends. Life Sci. 1991;49(10):689–705. doi: 10.1016/0024-3205(91)90101-g. [DOI] [PubMed] [Google Scholar]
- Fager G., Camejo G., Olsson U., Ostergren-Lundén G., Bondjers G. Heparin-like glycosaminoglycans influence growth and phenotype of human arterial smooth muscle cells in vitro. II. The platelet-derived growth factor A-chain contains a sequence that specifically binds heparin. In Vitro Cell Dev Biol. 1992 Mar;28A(3 Pt 1):176–180. doi: 10.1007/BF02631088. [DOI] [PubMed] [Google Scholar]
- Files J. C., Malpass T. W., Yee E. K., Ritchie J. L., Harker L. A. Studies of human plate alpha-granule release in vivo. Blood. 1981 Sep;58(3):607–618. [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Handin R. I. Vascular permeation of platelet factor 4 after endothelial injury. Science. 1980 Aug 1;209(4456):611–612. doi: 10.1126/science.6994228. [DOI] [PubMed] [Google Scholar]
- Handin R. I., Cohen H. J. Purification and binding properties of human platelet factor four. J Biol Chem. 1976 Jul 25;251(14):4273–4282. [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 1993 Aug;122(4):933–940. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Nishimura J., Huang S. S., Deuel T. F. Protamine inhibits platelet derived growth factor receptor activity but not epidermal growth factor activity. J Cell Biochem. 1984;26(4):205–220. doi: 10.1002/jcb.240260402. [DOI] [PubMed] [Google Scholar]
- Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993 Mar 26;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- Kaplow J. M., Bellot F., Crumley G., Dionne C. A., Jaye M. Effect of heparin on the binding affinity of acidic FGF for the cloned human FGF receptors, flg and bek. Biochem Biophys Res Commun. 1990 Oct 15;172(1):107–112. doi: 10.1016/s0006-291x(05)80179-2. [DOI] [PubMed] [Google Scholar]
- Khachigian L. M., Chesterman C. N. Synthetic peptides representing the alternatively spliced exon of the platelet-derived growth factor A-chain modulate mitogenesis stimulated by normal human serum and several growth factors. J Biol Chem. 1992 Apr 15;267(11):7478–7482. [PubMed] [Google Scholar]
- Khachigian L. M., Owensby D. A., Chesterman C. N. A tyrosinated peptide representing the alternatively spliced exon of the platelet-derived growth factor A-chain binds specifically to cultured cells and interferes with binding of several growth factors. J Biol Chem. 1992 Jan 25;267(3):1660–1666. [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Olson N. E., Clowes A. W., Reidy M. A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992 Nov;90(5):2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe M., Marshall S., Pepper D. S., Holbrook J. J. The transfer of platelet factor 4 from its proteoglycan carrier to natural and synthetic polymers. Biochim Biophys Acta. 1981 Nov 18;678(1):137–142. doi: 10.1016/0304-4165(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici I., Di Martino A., Cella G., Callegaro L., Prosdocimi M. Improved method for purification of human platelet factor 4 by affinity and ion-exchange chromatography. Thromb Res. 1989 May 15;54(4):277–287. doi: 10.1016/0049-3848(89)90086-8. [DOI] [PubMed] [Google Scholar]
- Meyer K., Smith R., Williams E. C. Inhibition of fibrin polymerization by serum amyloid P component and heparin. Thromb Haemost. 1987 Jun 3;57(3):345–348. [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Joseph-Silverstein J., Rifkin D. B. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol. 1986 Nov;129(2):273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987 Oct;105(4):1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Localization of thrombospondin in clots formed in situ. Blood. 1985 Nov;66(5):1098–1104. [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D., Dodge L., Fujii D. K. Heparin modulation of the neurotropic effects of acidic and basic fibroblast growth factors and nerve growth factor on PC12 cells. J Cell Physiol. 1987 Apr;131(1):131–140. doi: 10.1002/jcp.1041310119. [DOI] [PubMed] [Google Scholar]
- Nugent M. A., Edelman E. R. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry. 1992 Sep 22;31(37):8876–8883. doi: 10.1021/bi00152a026. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1992 Aug;118(3):631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Ross R. Rous-Whipple Award Lecture. Atherosclerosis: a defense mechanism gone awry. Am J Pathol. 1993 Oct;143(4):987–1002. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rybak M. E., Gimbrone M. A., Jr, Davies P. F., Handin R. I. Interaction of platelet factor four with cultured vascular endothelial cells. Blood. 1989 May 1;73(6):1534–1539. [PubMed] [Google Scholar]
- Sato Y., Abe M., Takaki R. Platelet factor 4 blocks the binding of basic fibroblast growth factor to the receptor and inhibits the spontaneous migration of vascular endothelial cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):595–600. doi: 10.1016/0006-291x(90)90715-y. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Waki M., Ohno M., Kuwano M., Sakata T. Carboxyl-terminal heparin-binding fragments of platelet factor 4 retain the blocking effect on the receptor binding of basic fibroblast growth factor. Jpn J Cancer Res. 1993 May;84(5):485–488. doi: 10.1111/j.1349-7006.1993.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Kaesberg P. R., Choay J., Harenberg J., Ershler W. B., Mosher D. F. Effects of sized heparin oligosaccharide on the interactions of Chinese hamster ovary cell with thrombospondin. Semin Thromb Hemost. 1992;18(2):243–251. doi: 10.1055/s-2007-1002430. [DOI] [PubMed] [Google Scholar]
- Sun X., Mosher D. F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989 Feb 15;264(5):2885–2889. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Tyrrell D. J., Ishihara M., Rao N., Horne A., Kiefer M. C., Stauber G. B., Lam L. H., Stack R. J. Structure and biological activities of a heparin-derived hexasaccharide with high affinity for basic fibroblast growth factor. J Biol Chem. 1993 Mar 5;268(7):4684–4689. [PubMed] [Google Scholar]
- Williams E. C., Huppert B. J., Asakura S. Neutralization of the anticoagulant effects of glycosaminoglycans by serum amyloid P component: comparison with other plasma and platelet proteins. J Lab Clin Med. 1992 Jul;120(1):159–167. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Katz I. R. Platelet factor 4: production, structure, and physiologic and immunologic action. Proc Soc Exp Biol Med. 1991 Nov;198(2):693–702. doi: 10.3181/00379727-198-43309. [DOI] [PubMed] [Google Scholar]


