Abstract
Background
Mechanisms responsible for anti-ischemic benefits of enhanced external counterpulsation (EECP) remain unknown. This was the first randomized, sham controlled study to investigate the extra-cardiac effects of EECP on peripheral artery flow mediated dilation.
Methods and Results
Forty-two symptomatic patients with coronary artery disease (CAD) were randomized (2:1 ratio) to either 35 1-hr sessions of EECP (n=28) or Sham-EECP (n=14). Flow-mediated dilation of the brachial and femoral arteries was performed using ultrasound. Plasma levels of nitrate and nitrite (NOx), 6-keto prostaglandin F1α (PGF1α), endothelin-1 (ET-1), asymmetric dimethylarginine (ADMA), tumor necrosis factor–α (TNF-α), monocyte chemoattractant protein–1 (MCP-1), vascular cell adhesion molecule (sVCAM), C-reactive protein (hsCRP), and 8-Isoprostane (8-iso-PGF2α) were measured. EECP increased brachial (+51% vs. +2%) and femoral (+30% vs. +3%) artery flow mediated dilation, the nitric oxide turnover/production marker NOx (+36% vs. +2%) and PGF1α (+71% vs. +1%), while decreasing ET-1 (-25% vs. +5%) and the nitric oxide synthase inhibitor ADMA (-28% vs. +0.2%) in treatment vs. sham, respectively (all p<0.05). EECP decreased the pro-inflammatory cytokines TNF-α (-16% vs. +12%), MCP-1 (-13% vs. +0.2%), sVCAM-1 (-6% vs. +1%), hsCRP (-32% vs. +5%), and the lipid peroxidation marker 8-iso-PGF2α (-21% vs. +1.3%) in treatment vs. sham, respectively (all p<0.05). EECP reduced angina classification (-62% vs 0%; p<0.001) in treatment vs. sham, respectively.
Conclusions
Our findings provide novel mechanistic evidence that EECP has a beneficial effect on peripheral artery flow mediated dilation and endothelial-derived vasoactive agents in patients with symptomatic CAD.
Keywords: angina, nitric oxide, endothelin, inflammation, vasodilation
INTRODUCTION
Enhanced external counterpulsation (EECP) is a non-invasive outpatient therapy for patients with chronic stable angina who fail to respond to standard revascularization procedures and aggressive pharmacotherapy. Data from the International Patient Registry (IEPR) demonstrate that EECP effectively decreases the frequency of angina episodes and nitrate usage, and increases exercise tolerance.1-3 However, the mechanism(s) responsible for the salutary clinical benefits of EECP remain largely unknown.
The central hypothesis, in most investigations conducted to elucidate the mechanism of action, is that EECP may promote coronary angiogenesis. However, this understanding of EECP is a theory and remains unconfirmed in randomized clinical trials.3, 4 In an international trial (7 Centers; 175 chronic stable angina patients), EECP failed to elicit improved cardiac perfusion in 46% of study subjects.5 In a recent US trial (6 US University Hospitals; 37 patients with stable chronic angina), EECP failed to improve myocardial blood flow to and within ischemic regions of the myocardium.6 However, despite negligible improvements in myocardial perfusion, approximately 85% of patients in EECP clinical trials experience reduction in angina.1, 5 To date, possible alternative extra-cardiac mechanisms associated with EECP have received little attention.7
Therefore, the purpose of the present study was to conduct the first randomized, sham-controlled investigation to assess the extra-cardiac effects of EECP on peripheral artery flow mediated dilation and endothelial-derived vasoactive agents in patients with refractory angina. Endothelial dysfunction is a systemic process and not necessarily confined to vascular beds with clinically overt atherosclerosis.8 We hypothesized that EECP would improve flow mediated dilation in peripheral muscular conduit arteries. We further hypothesized that EECP would elicit commensurate changes in endothelial-derived vasoactive agents, plasma inflammatory cytokines, and indices of oxidative damage. We reasoned that arterial flow mediated dilation and the balance of endothelial-derived vasoactive agents could be improved by enhanced hemodynamic shear stress with each inflation/deflation cycle of the compressive cuffs.9
METHODS
Baseline Status of Subjects
Forty-two consecutive patients with chronic stable angina referred for EECP treatment were randomized in a 2 : 1 manner into either an EECP treatment group or a Sham-EECP control group. The patients were enrolled for EECP treatment between November 2004 and June 2009 because they experienced chronic angina for more than 3 months caused by myocardial ischemia in the presence of angiographic multivessel coronary artery disease that could not be controlled by a combination of medical therapy, angioplasty/stent, and/or coronary bypass surgery. The study was approved by the Institutional Review Board of the University of Florida and written informed consent was obtained from all patients.
Exclusion Criteria
Absence of ST segment depression (1 mm minimum) during exercise testing, > 75 years of age, coronary artery bypass graft (CABG) within past 3 months or PCI in past 6 months, cardiac catheterization for any reason within past 2 weeks, arrhythmia that would significantly interfere with triggering of the EECP device, symptomatic heart failure and/or LV ejection fraction < 30%, valvular heart disease, ICD if triggered within past 6 months, history of deep vein thrombosis, uncontrolled hypertension, pregnancy, pulmonary congestion, and systemic hypotension.
EECP and Sham
Patients in the EECP (n = 28) and Sham (n = 14) groups received 35 1-h daily sessions of EECP for 7 consecutive weeks using cuff inflation pressures of 300 and 70 mm Hg, respectively. It was previously determined that 70 mm Hg inflation pressure for Sham is adequate to preserve the appearance and feel of EECP application, but insufficient to alter blood pressure.1 EECP equipment (Vasomedical, Westbury, New York) has been described previously.1
Blood Collection and Biochemical Assays
Venipuncture was performed before and after 35 sessions of EECP or Sham. Plasma levels of tumor necrosis factor–α (TNF-α), monocyte chemoattractant protein–1 (MCP-1), soluble vascular cell adhesion molecule–1 (sVCAM-1), and high sensitivity C-reactive protein (hsCRP) were determined by enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D Systems Inc., Minneapolis, Minnesota). The intra- and inter assay coefficients of variance were 4.6% and 7.7% for TNF-α, 4.2% and 6.9% for MCP-1, 3.4% and 6.1% for sVCAM-1, and 4.6% and 8.0% for CRP, respectively. Serum lipids and glucose were measured in hospital laboratories by validated techniques.
Measurement of the stable nitric oxide metabolites, nitrate and nitrite (NOx), was used to estimate nitric oxide production. Patients kept a diet diary and followed the National Institutes of Health low nitrate diet guidelines a minimum of 48 hours prior to each blood draw.10 NOx, 6-keto prostaglandin F1α (6-keto-PGF1α) the major metabolite of prostacyclin, and plasma superoxide dismutase (SOD), an index of total endogenous antioxidant capacity, were measured by commercial assays (Cayman, Ann Arbor, MI). ELISA kits were also used to measure plasma levels of endothelin-1 (ET-1)(Quantikine, Minneapolis, MN), 8-Isoprostane (8-iso-PGF2α) as an index of total oxidative stress (Assay Designs, Ann Arbor, MI), norepinephrine, and the endogenous endothelial nitric oxide synthase competitive inhibitor asymmetric dimethylarginine (ADMA)(Alpco, Salem, NH) The intra- and inter assay coefficients of variance were 3.2% and 5.0% for NOx, 5.5% and 8.1% for 6-keto-PGF1α, 4.3% and 7.0% for SOD, 3.4% and 7.6% for ET-1, 1.3% and 2.2% for 8-iso-PGF2α, 4.2% and 7.1% for norepinephrine, and 5.3% and 7.2% for ADMA. Biochemical assays were performed in 2 batches; once at the midpoint and after completion of the study.
Peripheral Flow Mediated Dilation (FMD)
The FMD technique was used to determine endothelial-dependent reactivity in the brachial and femoral arteries before and after 35 sessions of EECP or Sham. Brachial artery FMD was assessed in the right arm using a high-resolution ultrasound machine (ATL HDI 3000; Advanced Technologies Laboratories, Bothell, WA) equipped with a 10.5MHz transducer.11 Briefly, resting baseline end diastolic brachial diameters and blood velocity were obtained with the transducer placed 3-5 cm above the anticubital fossa. Reactive hyperemia was produced by inflating a BP cuff placed on the upper forearm for 5 minutes at 200 mmHg followed by a rapid deflation. The brachial artery was imaged and recorded for 3 minutes following cuff deflation. Ultrasound images were recorded on a super VHS videocassette for off-line electronic image analysis using Image Pro Software (Image Pro, Data Translation, Inc., Marlboro, MA). Brachial artery diameters were determined during end-diastole (gated with electrocardiogram R wave) by measuring the distance between the near and far wall of the intima. Peak brachial FMD was expressed as a percentage increase from baseline (FMD%). FMD% is influenced by baseline diameter so absolute changes (Δmm) in diameter were also determined.12 Brachial measurements were normalized to the mean shear rate calculated from the first 10 seconds following cuff dilation. Similarly, femoral artery FMD was performed in the right common femoral artery 2 to 3 cm proximal to the bifurcation. Cuff placement was distal to the arterial imaging site 5 cm above the patella. The cuff was inflated for 5 minutes at 200 mmHg followed by rapid deflation. All brachial and femoral artery FMD procedures were performed in the Clinical Exercise Physiology Laboratory at the University of Florida by 2 experienced ultrasound technicians who had undergone previous training on this technique. The ultrasound images were measured blind to treatment allocation and stage of FMD test.
Graded Exercise Tests
All subjects performed symptom limited maximum graded exercise tests (SL-GXT) on a treadmill using a modified Naughton protocol before and after 35 sessions of EECP or Sham. Primary measurements included time to angina, total exercise duration, and VO2peak. Criteria for termination of the SL-GXT included 2.5 - 3 mm ST depression, respiratory exchange ratio (RER) > 1, angina = 2.5 – 3 on a 4 point scale, plateau of VO2, and volitional fatigue.
Statistical Analysis
All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, Illinois). Continuous variable data are presented as mean ± SD. All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of p < 0.05 was required for statistical significance. Repeated measures analysis of variance (ANOVA) was used to evaluate the continuous primary dependent variables, brachial and femoral artery FMD; and the secondary dependent variables, NOx, ET-1; the plasma biomarkers, patient characteristics, and all other data. When a statistically significant main effect between treatment and Sham was determined, within group repeated measures ANOVA’s were performed for each variable to analyze the timepoint mean differences from baseline for each group and to determine within group timepoint significance. Further, Tukey post hoc analysis was performed utilizing the within subject timepoint effect mean square error and test of between subject effect mean square error derived from the primary repeated measures ANOVA.
When significant differences between timepoints were observed for the EECP group, and there were no other significant differences between groups at baseline or between timepoints within the Sham group, the significant p values for the absolute mean changes are reported within group by timepoint to simplify the presentation of the EECP treatment effect. Statistically significant absolute values are represented in the figures as percent changes from baseline and Tukey’s post hoc significance between group between timepoint are reported. Repeated measures ANOVA between treatment and Sham was used to analyze the descriptive patient characteristics, metabolic profile, cardiac intervention history, drug regimens, and graded exercise test results. These data are reported as mean ± SD.
Sample Size Calculation
A power analysis was performed to estimate the statistical power related to testing the following hypotheses in 30 patients: 1) EECP would improve endothelial function in peripheral muscular conduit arteries measured via flow mediated dilation of the brachial artery. 2) EECP would elicit commensurate changes in endothelial-derived vasoactive agent NOx. Based on the data of Shechter et al.13, for Hypothesis 1 the post treatment means were anticipated to be 8.2% and 3.1% for EECP and Sham control respectively. The anticipated standard deviation was about 2.1% for the EECP group and 2.2% for the Sham control group. A study of 20 evaluable EECP subjects and 10 evaluable Sham controls will have 99% power, based on the Satterthwaite corrected t-test to have a P-value below 5% two-sided. Based on the data of Akhtar et al.14, for Hypothesis 2 the post treatment means are anticipated to be 43.9 and 27.1 μmol/L for EECP and Sham control respectively. The anticipated standard deviation was about 7.5 μmol/L for the EECP group and 4.75 μmol/L for the Sham control group. A study of 20 evaluable EECP subjects and 10 evaluable Sham controls will have 99% power, based on the Satterthwaite corrected t-test to have a P-value below 5% two-sided.
RESULTS
All patients completed the entire EECP treatment or Sham regimen and there were no adverse cardiovascular events. Table 1 contains the patient descriptive characteristics and metabolic profile. Table 2 contains cardiac intervention history and drug regimens. There were no differences between the 2 groups at study entry with respect to blood pressure, drug therapy, previous cardiovascular history, cardiovascular risk factors, revascularization procedures, and metabolic profile. The majority were men (81%) and all had a long history of CAD and interventions, and were not candidates for further revascularization therapy. All patients were taking at least 2 anti-angina drugs, and the majority (86%) were taking 3. Medications were not changed during the treatment period and all patients waited until completion of the study before initiating exercise regimens that differed from activity levels at study entry. All vasodilator drugs were discontinued 12 hours before laboratory testing. Four patients in EECP (14%) and 2 patients in Sham (14%) were insulin-dependent diabetics. None of the patients were current smokers.
Table 1.
Patient descriptive characteristics and metabolic profile.
| EECP (N=28) | SHAM (N=14) | P | |||
|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||
| Age (years) | 64.44±9.63 | 64.57±9.63 | 64.27±10.38 | 64.40±10.38 | .906 |
| Height (cm) | 172.77±21.61 | 172.77±21.61 | 173.99±8.34 | 173.99±8.34 | .360 |
| Weight (kg) | 92.60±17.24 | 92.42±17.32 | 100.81±12.48 | 100.29±12.89 | .518 |
| BMI (kg/m2) | 30.88±11.15 | 30.80±11.02 | 33.37±4.24 | 33.19±4.34 | .460 |
| Glucose (mg/dL) | 121.46±46.76 | 144.62±92.29 | 138.38±57.94 | 145.86±50.85 | .973 |
| Triglycerides (mg/dL) | 148.42±70.89 | 176.08±101.57 | 162.33±79.84 | 154.89±92.72 | .263 |
| Total Cholesterol (mg/dL) | 142.69±37.37 | 142.08±23.86 | 144.33±36.78 | 141.56±31.77 | .829 |
| LDL (mg/dL) | 69.83±33.43 | 67.83±28.89 | 74.22±30.16 | 72.56±25.68 | .962 |
| HDL (mg/dL) | 39.31±16.36 | 37.08±16.20 | 37.67±5.81 | 38.11±5.78 | .379 |
There were no significant differences (p>0.05) in patient descriptive characteristics or metabolic profile between EECP and Sham groups at baseline or after treatment. Data are expressed as mean
Table 2.
Baseline patient cardiac intervention history and drug regimens.
| EECP (N=28) | SHAM (N=14) | P | |
|---|---|---|---|
| Prior CABG | 19 (76%) | 11 (79%) | .481 |
| Prior PTCA | 23 (92%) | 12 (88%) | .776 |
| Prior Myocardial Infarction | 17 (61%) | 6 (43%) | .284 |
| Multivessil CAD | 25 (89%) | 13 (93%) | .718 |
| Diabetes | 21 (84%) | 11 (79%) | .804 |
| Hypertension | 23 (92%) | 12 (88%) | .776 |
| Hyperlipidemia | 26 (93%) | 14 (100%) | .317 |
| Lipid Lowering | 28 (100%) | 14 (100%) | .999 |
| Beta-Blocker | 24 (86%) | 11 (79%) | .569 |
| Calcium Channel Blocker | 11 (39%) | 6 (43%) | .829 |
| Long Lasting NItrates | 25 (89%) | 12 (88%) | .744 |
| ACE Inhibition (or)ARB | 26 (93%) | 12 (88%) | .469 |
| Insulin | 9 (32%) | 5 (36%) | .822 |
There were no significant differences (p>0.05) in baseline characteristics, drug regimens, and cardiac intervention history between SHAM and EECP group at baseline. Values are presented as the number of patients per group and the percentage within each group.
EECP Improved Brachial and Femoral Artery Flow Mediated Dilation
At study entry, resting diameter and FMD (absolute diameter and Δ%) of the brachial and femoral arteries did not differ between groups. EECP therapy improved absolute brachial artery FMD (Figure 1A), percent change in dilation (Figure 1C), and FMD normalized during first 10 seconds following cuff release (0.18 ± 0.05 to 0.30 ± 0.11 and 0.20 ± 0.07 to 0.21 ± 0.07 Normalized s-1 in EECP and Sham, respectively; p < 0.01 treatment vs. Sham). EECP improved absolute femoral artery FMD (Figure 1B) and percentage change in dilation (Figure 1D). No changes in brachial or femoral FMD occurred in the Sham group.
Figure 1.
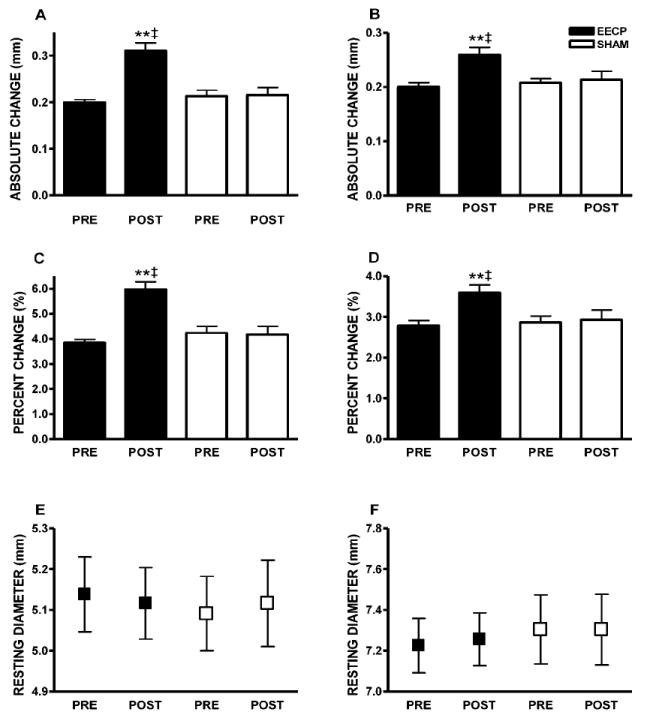
Data are absolute values from within group repeated measures ANOVA and Tukey post hoc analysis of between group and between timepoint differences in absolute values. (A) Brachial artery FMD diameter (mm); (B) femoral artery FMD diameter (mm); (C) brachial artery FMD (Δ%); and (D) femoral artery FMD (Δ%); (E) brachial artery absolute diameter; and (F) femoral artery absolute diameter before (PRE) and after (POST) 35 sessions of EECP. **p < 0.01 versus pretreatment values; ‡p < 0.01 EECP versus SHAM. Data are expressed as mean ± SEM.
EECP Increased Plasma NOx, 6-keto-PGF1α, and Decreased ET-1
At study entry, plasma levels of NOx, 6-keto-PGF1α, and ET-1 did not differ between groups. EECP treatment increased NOx (22.8 ± 9.8 to 31.9 ± 11.1 and 23.9 ± 16.1 to 24.3 ± 14.5 μmol/L in EECP and Sham, respectively; p < 0.01 treatment vs. Sham; Figure 2A), while ET-1 levels were concurrently decreased (2.2 ± 0.8 to 1.5 ± 1.3 and 2.1 ± 0.9 to 2.1 ± 0.9 pg/ml in EECP and Sham, respectively; p < 0.05 treatment vs. Sham; Figure 2B). Consequently, the NOx : ET-1 ratio was improved (16.8 ± 4.8 to 26.5 ± 5.6 and 13.3 ± 3.3 to 12.3 ± 2.8 in EECP and Sham, respectively; p < 0.01 treatment vs. Sham; Figure 2C) after EECP. The vasodilatory prostaglandin 6-keto-PGF1α increased (108 ± 71 to 185 ± 111 and 118 ± 60 to 120 ± 94 pg/ml in EECP and Sham, respectively; p < 0.05 treatment vs. Sham; Figure 2D) after EECP. Plasma levels of NOx, 6-keto-PGF1α, ET-1, and the NOx : ET-1 ratio did not change in the Sham group.
Figure 2.
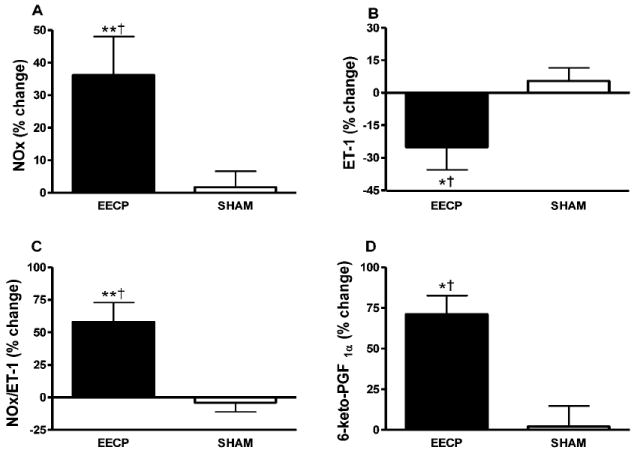
Statistically significant absolute values are represented as percent change from baseline. P values are from within group repeated measures ANOVA and Tukey post hoc analysis of between group and between timepoint differences in absolute values. Percentage (%) change in plasma (A) NOx; (B) ET-1; (C) NOx/ET-1 ratio; and (D) 6-keto-PGF1α after 35 sessions of EECP. *p < 0.05 versus pretreatment values; **p < 0.01 versus pretreatment values; †p < 0.05 EECP versus SHAM. Data are expressed as mean ± SEM.
EECP Decreased Inflammatory Cytokines and Endothelial Adhesion Molecules
At study entry, plasma levels of TNF-α, hsCRP, MCP-1, and sVCAM did not differ between groups. EECP therapy decreased TNF-α (6.1 ± 2.2 to 5.0 ± 1.7 and 6.2 ± 1.2 to 6.4 ± 1.5 pg/ml in EECP and Sham, respectively; p < 0.01 treatment vs. Sham; Figure 3A), hsCRP (3.4 ± 2.17 to 2.3 ± 1.8 and 3.4 ± .9 to 3.5 ± 1.1 mg/L in EECP and Sham, respectively; p < 0.05 treatment vs. Sham; Figure 3B), MCP-1 (227 ± 74 to 171 ± 43 and 230 ± 92 to 231 ± 75 pg/ml in EECP and Sham, respectively; p < 0.001 treatment vs. Sham; Figure 3C), and sVCAM (917 ± 406 to 821 ± 317 and 955 ± 256 to 967 ± 230 ng/ml in EECP and Sham, respectively; p < 0.05 treatment vs. Sham; Figure 3D). No changes in inflammatory cytokines or endothelial adhesion molecules were observed in the Sham group.
Figure 3.
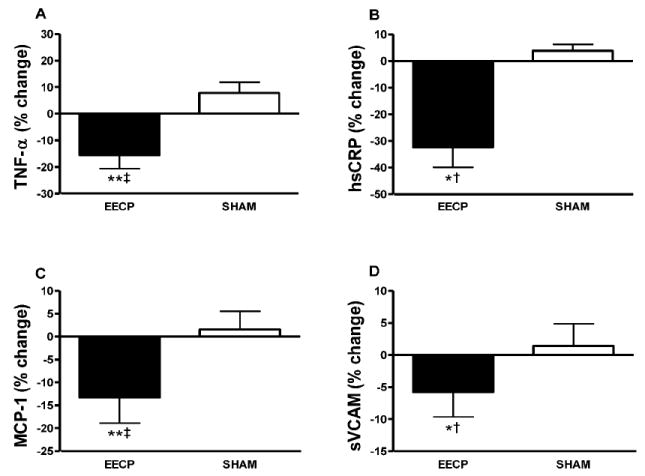
Statistically significant absolute values are represented as percent change from baseline. P values are from within group repeated measures ANOVA and Tukey post hoc analysis of between group and between timepoint differences in absolute values. Percentage (%) change in plasma (A) TNF-α; (B) hsCRP; (C) MCP-1; and (D) sVCAM after 35 sessions of EECP. *p < 0.05 versus pretreatment values; **p < 0.01 versus pretreatment values; †p < 0.05 EECP versus SHAM; ‡p < 0.01 EECP versus SHAM. Data are expressed as mean ± SEM.
EECP Improved Redox Balance
At study entry, plasma levels of 8-iso-PGF2α, ADMA, and SOD did not differ between groups. EECP decreased 8-iso-PGF2α (1,709 ± 594 to 1,356 ± 411 and 1,720 ± 674 to 1,696 ± 472 pg/ml in EECP and Sham, respectively; p < 0.01 treatment vs. Sham; Figure 4A) and ADMA (0.64 ± 0.18 to 0.46 ± 0.18 and 0.66 ± 0.11 to 0.66 ± 0.22 μmol/ml in EECP and Sham, respectively; p < 0.001 treatment vs. Sham; Figure 4B). SOD did not change significantly after EECP (1.59 ± 0.39 to 1.49 ± 0.48 and 1.54 ± 0.68 to 1.46 ± 0.66 U/ml in EECP and Sham, respectively; p > 0.05 treatment vs. Sham; Figure 4C). Levels of 8-iso-PGF2α, ADMA, and SOD did not change in the Sham group.
Figure 4.
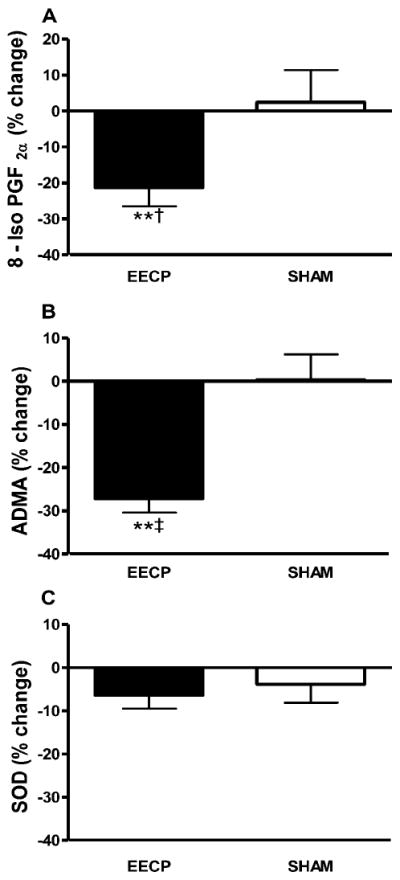
Statistically significant absolute values are represented as percent change from baseline. P values are from within group repeated measures ANOVA and Tukey post hoc analysis of between group and between timepoint differences in absolute values. Percentage (%) change in plasma (A) 8-Iso PGF2a; (B) ADMA; and (C) SOD after 35 sessions of EECP. **p < 0.01 versus pretreatment values; †p < 0.05 EECP versus SHAM; ‡p < 0.01 EECP versus SHAM. Data are expressed as mean ± SEM.
EECP Decreased Peripheral Blood Pressure
Peripheral blood pressure and heart rate information is presented in Table 1. We observed no significant changes in resting heart rate in either group. Resting systolic and diastolic brachial pressures were decreased after 35 sessions of EECP. No changes in blood pressure were observed in the sham group.
EECP Increased VO2peak, Exercise Duration and Time to Angina
At study entry, time to angina, VO2peak, total exercise duration, and peak respiratory exchange ratio (RER), heart rate, systolic blood pressure and rating of perceived exertion (RPE) did not differ between groups during the SL-GXT (Table 3). After 35 sessions of EECP, time to angina, VO2peak, and total exercise duration were increased. No changes in SL-GXT parameters were observed in the Sham group.
Table 3.
Results from the symptom-limited graded exercise tests (SL-GXT).
| EECP (n=28) | SHAM (n=14) | ||||
|---|---|---|---|---|---|
| T1 (M ± SD) | T2 (M ± SD) | T1 (M ± SD) | T2 (M ± SD) | P, Between Group Comparison | |
| Peak VO2 (ml/kg/min) | 17.0±6.0 | 19.4±6.9† | 16.5±4.7 | 16.6±5.2 | 0.002 |
| Peak Exercise Duration (s) | 586.0±193.5 | 773.6±263.2† | 597.1±181.6 | 612.1±175.6 | < 0.001 |
| Peak Time to Angina (s) | 406.1±184.6 | 645.1±297.8‡ | 449.4±202.9 | 471.3±197.4 | 0.003 |
| Peak Angina Rating | 2.5±0.9 | 1.7±1.1† | 2.3±1.2 | 2.3±1.3 | 0.045 |
| Peak Heart Rate (bpm) | 112.4±13.8 | 117.4±18.9 | 115.6±20.1 | 119.1±19.1 | 0.550 |
| Rating of Perceived Exertion | 16.1±1.8 | 16.8±1.9 | 16.6±1.7 | 16.4±1.6 | 0.249 |
| Respiratory Exchange Ratio | 0.99±0.08 | 1.04±0.08 | 0.98±0.12 | 0.98±0.10 | 0.182 |
There were no significant differences between EECP and SHAM groups at baseline. Data are presented as mean ± SD. Significance values are also reported from within group repeated measures ANOVA and Tukey post hoc analysis;
p < 0.01,
p < 0.001.
EECP Improved Functional Classification
EECP led to an improvement by 1 CCS class in 2 patients (7%), by 2 classes in 19 patients (68%), and by 3 classes in 6 patients (21%). One patient did not change CCS class. Average CCS classification (3.16 ± 0.47 to 1.20 ± 0.40 and 2.93 ± 0.26 to 2.93 ± 0.26 in EECP and Sham, respectively; p < 0.001 treatment vs. Sham), number of angina episodes per day (1.8 ± 1.47 to 0.5 ± 0.70 and 1.7 ± 1.39 to 1.6 ± 1.24 in EECP and Sham, respectively; p < 0.01 treatment vs. Sham), and daily nitrate usage (1.1 ± 1.44 to 0.2 ± 0.41 and 1.0 ± 1.12 to 0.9 ± 1.11 in EECP and Sham, respectively; p < 0.01 treatment vs. Sham) decreased in the EECP group. There were no changes in any measure of angina symptom reduction in the Sham group.
EECP did not change Autonomic Nervous System Activation or Metabolic Profile
At study entry, plasma levels of NE did not differ between groups. We observed no change in plasma norepinephrine after 35 sessions of EECP (908 ± 129 to 885 ± 196 pg/ml) or Sham (902 ± 107 to 914 ± 98 pg/ml). None of the metabolic profile variables were different between groups at study entry (Table 1). Serum glucose, triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol levels did not change in either group.
DISCUSSION
This prospective, randomized, Sham-controlled study demonstrates for the first time in the same cohort of CAD patients, that EECP intervention: (1) improves brachial and femoral artery flow mediated dilation, (2) increases bioavailability of nitric oxide and PGF1, (3) decreases plasma biomarkers of inflammation, oxidative stress, and ET-1, (4) decreases angina symptoms, and (5) increases exercise tolerance. These EECP-induced clinical benefits occurred in CAD patients with refractory angina pectoris who were receiving optimal medical therapy.
Flow mediated vasodilation
The primary focus of the present study was the direct effects of episodic changes in shear stress, as delivered during repeated bouts of EECP, on peripheral muscular conduit artery FMD. Flow mediated dilation in the brachial and femoral arteries was increased by 51% and 30%, respectively (Figure 1). These arterial adaptations appear to be the collective result of our observed dramatic increases in NOx and PGF1, and concurrent decreases in the vasoconstrictor ET-1.
Endothelial-derived vasoactive agents
Endothelial dysfunction in patients with CAD is manifest by either decreased secretion of paracrine vasodilators including nitric oxide and PGF1, or an increased production of the potent vasoconstrictor ET-1, and/or resistance of vascular smooth muscle to nitric oxide. In the present study, 35 sessions of EECP increased plasma levels of NOx by 36%, whereas ET-1 levels were concurrently decreased by 25% (Figure 2). ET-1 values after EECP treatment compare favorably with reference values (1 to 1.5 pg/ml) in normal healthy humans.15, 16 Previously, Zhang and co-workers17 reported that EECP increased the expression of endothelial nitric oxide synthase in hypercholesterolemic pigs, and small uncontrolled studies have also reported increased plasma NOx levels in coronary artery disease patients after EECP.4, 14 Akhtar et al.14 found that NOx remained significantly above baseline (+12%) and ET-1 remained significantly below baseline (-11%) at 3 months after completion of EECP therapy in CAD patients.
This was the first study to examine the mitigating effects of EECP on circulating levels of the potent endothelial-derived vasodilator PGF1, the stable metabolite of prostacyclin. We found a 171% increase in PGF1 following EECP (Figure 2). This was also the first study to measure the effects of EECP on plasma levels of asymmetric dimethylarginine (ADMA), the major endogenous competitive inhibitor of nitric oxide synthase. We observed a 28% reduction in plasma ADMA levels following EECP (Figure 3). This finding is similar to what has been reported following exercise training in Type 1 diabetics18 and CAD patients.19 ADMA can be increased up to 10-fold greater than normal in patients with CAD.20 ADMA contributes to endothelial dysfunction by displacing the substrate L-arginine from the catalytic site of nitric oxide synthase, thus inhibiting nitric oxide formation and reducing nitric oxide bioavailability. Oxidative stress is a key modulator of ADMA levels,20 suggesting that our observed 21% reduction in the marker of oxidative stress (8-iso-PGF2α; Figure 3) may have led to decreased levels of ADMA.
Mechanisms for endothelial-derived vasoactive agents
The mechanism responsible for increased NOx and PGF1, and concurrent decreases in plasma ET-1 and 8-iso-PGF2α following EECP therapy is likely related to the intermittent bouts of shear stress created with each inflation-deflation cycle of the pneumatic cuffs. Shear stress is a primary stimulus for the synthesis and release of endothelial-derived nitric oxide.17 The shear stress stimulus is transduced into the endothelial cell via the integrin/cytoskeleton mechano-transduction pathway.9, 21 Sequential inflation (~ 300 mm mercury) of the 3 pneumatic EECP cuffs from calf to buttocks during diastole produces a robust retrograde pressure wave in the central aorta that subsequently increases coronary artery perfusion pressure, analogous to intraaortic balloon counterpulsation.22 Possible clinical benefits of EECP-induced coronary diastolic augmentation have been studied extensively in patients with CAD.2-6, 23-25
However, inflation of the EECP cuffs also produces high-pressure retrograde blood flow in the femoral arteries and simultaneous moderate-pressure antegrade flow in the brachial arteries.17, 26 Using color Doppler imaging in a porcine EECP model, Zhang and coworkers17 found that brachial artery blood flow velocity increased by 132% (59 versus 24 cm/second; p < 0.001) and brachial artery wall shear stress increased by > 200% (49 versus 23 dyne/cm2, p < 0.001) during lower body compression of EECP pneumatic cuffs. Currently there are few therapeutic interventions that could create episodic increases in arterial shear stress in humans. Selected modes of exercise are known to create pulsatile, hemodynamic shear forces and improve endothelial function.27 However, in CAD patients with refractory angina, exercise tolerance is often below the minimum metabolic threshold necessary to elicit exercise training effects.28 Our data suggest that the significant increases in pulsatile and oscillatory flow during EECP treatment, as recorded by color Doppler, may provide a form of “massage” on the endothelium and improve its function. That is, each session of EECP may be thought of as providing a direct dose of vascular medicine.
Plasma markers of inflammation
Endothelial function is adversely affected by inflammation, and there is extensive evidence indicating that patients with CAD have elevated levels of proinflammatory cytokines and adhesion molecules,29 including TNF-α, hsCRP, MCP-1, and sVCAM. Moreover, CAD patients with refractory angina symptoms have proinflammatory biomarker levels that are elevated even further.30 Increased plasma levels of TNF-α, hsCRP, and MCP-1 are known to predict future coronary events. In vitro, CRP and TNF-α also actively participate in the atherogenic process by contributing to decreased nitric oxide synthase production and increased endothelial cell activation characterized by expression of sVCAM. After EECP, we observed reductions in plasma levels of TNF-α (- 16%), hsCRP (- 32%), MCP-1 (- 13%), and sVCAM (- 6%) (Figure 4). The anti-inflammatory effects of EECP may be clinically relevant with regard to decreasing the risk for future cardiovascular events in this patient population. For instance, sVCAM levels above ~1130 ng/ml are associated with increased ischemic events and mortality in patients with CAD.31, 32 In the present study, 6 of 28 patients in the intervention group had baseline sVCAM > 1130 ng/ml. After EECP, only 1 of 28 patients had sVCAM values > 1130 ng/ml. Similarly, EECP shifted mean CRP values from the high- risk (> 3 mg/L) to the moderate-risk category (1 to 3 mg/L).33 Despite significant reductions, TNFα levels after EECP remained high and approximated the 95th percentile of healthy control values.34 Increased bioavailability of nitric oxide after EECP therapy is the likely mechanism responsible for reduced plasma inflammatory markers. Nitric oxide serves an anti-inflammatory role by inhibiting the expression of MCP-1 and reducing VCAM-1 expression.35
Lipid peroxidation and antioxidant capacity
This was the first study to examine the effects of EECP on lipid peroxidation. We observed a 21% decrease in plasma levels of 8-iso-PGF2a (Figure 4). F2-isoprostanes are a class of prostaglandin-like compounds that are produced by free radical-mediated lipid peroxidation of arachidonic acid, independent of cyclooxygenase. One of these isoprostanes, 8-iso-PGF2a, is viewed as the most valid plasma marker to assess systemic oxidative stress. 8-iso-PGF2a is a potent vasoconstrictor and an independent risk factor associated with CAD.36 The mechanisms responsible for decreased 8-iso-PGF2a are unclear because EECP did not change activity levels of the extracellular antioxidant enzyme SOD (Figure 4). However, nitric oxide has been shown to have antioxidative properties and our observed 36% increase in plasma NOx following EECP could suppress oxidative stress.
Angina symptoms
Our evidence of improved arterial FMD and endothelial-derived vasoactive agents after EECP was paralleled by decreased angina symptoms. Patients reduced their CCS angina classification from 3.1 to 1.2 (mean ± SEM), daily angina episodes by approximately 72%, and daily nitrate usage by 81%. The anti-angina benefits of EECP are perhaps best illustrated by the fact that 7 patients in the intervention group completed their exit SL-GXT without any symptoms of angina. In contrast, each of those 7 patients experienced angina levels between 2 and 3 (on the 4 point scale) on their SL-GXT at study entry.
Clinical Relevance
We believe that the present findings have important clinical relevance. In the US alone, there are over 2.5 million patients with symptomatic CAD that are not amenable to percutaneous coronary intervention or bypass surgery, because of unsuitable coronary anatomy, multiple previous revascularization attempts, age, additional co-morbid conditions, or patient preference.37 For those patients in whom repeat (or initial) revascularization procedures are not appropriate, and in whom aggressive medical therapy fails to reduce anginal pain, the search for alternate therapeutic options continues. EECP is the only truly noninvasive, atraumatic intervention for which a reduction of angina symptoms and nitrate usage, increased exercise tolerance, and an improvement in myocardial ischemia have been shown.
Study Limitations
Reduced sympathetic tone38 during the 35 sessions of EECP could independently improve peripheral artery reactivity. However, our plasma norepinephrine results indicate that sympathetic tone was unchanged after 35 sessions of EECP. Changes in metabolic profile could also confound the findings of the present study. However, we found no differences in lipid profile between groups at study entry, and there were no changes in total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and glucose levels during the 7 to 8 weeks of the study (Table 1). Lastly, we did not perform endothelium-independent reactivity tests. However, it is unlikely that our observed improvements in FMD and limb blood flow are due to heightened smooth muscle sensitivity to nitric oxide or altered cyclic guanosine monophosphate signaling during EECP treatment. Indeed, it was previously reported that effects of sublingual nitroglycerine spray, a nitric oxide donor acting directly on smooth muscle cells, are unchanged in CAD patients after EECP therapy.39
Summary
In summary, by demonstrating that 35 1-hr sessions of EECP improves brachial and femoral artery FMD, results from the present Sham-controlled study support the hypothesis that EECP improves peripheral arterial function in patients with symptomatic CAD. Our hypothesis is further supported by novel neurohormonal evidence that EECP markedly increases plasma NOx and 6-keto-PGF1α, and simultaneously decreases ET-1, 8-iso-PGF2α, and the pro-inflammatory markers TNF-α, hsCRP, MCP-1, and sVCAM.
Clinical Summary.
Not all patients with symptomatic coronary artery disease (CAD) are amenable to standard revascularization procedures because of unsuitable coronary anatomy, multiple previous revascularization attempts, age, additional co-morbid conditions, or patient preference. Such difficult cases require physicians to entertain other options. In the U.S. alone, there are over 2.4 million patients with CAD not amenable to bypass or angioplasty. For those patients in whom repeat (or initial) revascularization procedures are not appropriate, and in whom aggressive medical therapy fails to reduce anginal pain, the search for additional therapeutic options continues. New treatment modalities, which are at various stages of clinical evaluation, include minimally invasive bypass surgery, spinal cord stimulation, transcutaneous electrical nerve stimulation, transmyocardial laser revascularization, and enhanced external counter pulsation (EECP). Of these modalities, EECP is the only truly noninvasive, atraumatic technique for which a reduction of anginal symptoms and nitrate use, increased exercise tolerance and an improvement in objective measures of myocardial ischemia have been shown. The present study was the first sham-controlled, randomized investigation to focus on peripheral vascular mechanisms that may contribute to the clinical benefits of EECP treatment. In a cohort of symptomatic coronary artery disease patients (n = 42), EECP reduced angina episodes, nitrate usage, and CCS angina classification. EECP elicited commensurate improvements in peripheral artery vasodilatory capacity and increases in plasma levels of endothelial-derived vasoactive agents. These novel findings support the hypothesis that EECP improves peripheral endothelial function in patients with symptomatic function and may reduce myocardial oxygen demand.
Acknowledgments
Funding Sources: This project was funded by the National Institutes of Health, NIH/HLB Grant #R01 HL077571 to Randy W. Braith, PhD
Footnotes
Conflict of Interest Disclosure. The authors have no conflicts of interest.
Disclosures: None
References
- 1.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. Journal of the American College of Cardiology. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 2.Lawson WE, Hui JC, Zheng ZS, Burgen L, Jiang L, Lillis O, Oster Z, Soroff H, Cohn P. Improved exercise tolerance following enhanced external counterpulsation: cardiac or peripheral effect? Cardiology. 1996;87:271–275. doi: 10.1159/000177103. [DOI] [PubMed] [Google Scholar]
- 3.Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. Journal of the American College of Cardiology. 2001;37:93–99. doi: 10.1016/s0735-1097(00)01095-0. [DOI] [PubMed] [Google Scholar]
- 4.Masuda D, Nohara R, Hirai T, Kataoka K, Chen LG, Hosokawa R, Inubushi M, Tadamura E, Fujita M, Sasayama S. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by(13)N-ammonia positron emission tomography. Eur Heart J. 2001;22:1451–1458. doi: 10.1053/euhj.2000.2545. [DOI] [PubMed] [Google Scholar]
- 5.Stys TP, Lawson WE, Hui JC, Fleishman B, Manzo K, Strobeck JE, Tartaglia J, Ramasamy S, Suwita R, Zheng ZS, Liang H, Werner D. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol. 2002;89:822–824. doi: 10.1016/s0002-9149(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 6.Michaels AD, Raisinghani A, Soran O, de Lame PA, Lemaire ML, Kligfield P, Watson DD, Conti CR, Beller G. The effects of enhanced external counterpulsation on myocardial perfusion in patients with stable angina: a multicenter radionuclide study. Am Heart J. 2005;150:1066–1073. doi: 10.1016/j.ahj.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 7.Bonetti PO, Holmes DR, Jr, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what’s behind the curtain? Journal of the American College of Cardiology. 2003;41:1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. The American journal of cardiology. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free radical biology & medicine. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 12.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 13.Shechter M, Matetzky S, Feinberg MS, Chouraqui P, Rotstein Z, Hod H. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. J Am Coll Cardiol. 2003;42:2090–2095. doi: 10.1016/j.jacc.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98:28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Tanzilli G, Barilla F, Pannitteri G, Greco C, Comito C, Schiariti M, Papalia U, Mangieri E. Exercise training counteracts the abnormal release of plasma endothelin-1 in normal subjects at risk for hypertension. Ital Heart J. 2003;4:107–112. [PubMed] [Google Scholar]
- 16.Maeda S, Miyauchi T, Iemitsu M, Sugawara J, Nagata Y, Goto K. Resistance exercise training reduces plasma endothelin-1 concentration in healthy young humans. Journal of cardiovascular pharmacology. 2004;44(Suppl 1):S443–446. doi: 10.1097/01.fjc.0000166319.91623.b0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, Du Z, Jin Y, Xiong Y, He J, Fang D, Wang K, Lawson WE, Hui JC, Zheng Z, Wu G. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation. 2007;116:526–534. doi: 10.1161/CIRCULATIONAHA.106.647248. [DOI] [PubMed] [Google Scholar]
- 18.Mittermayer F, Pleiner J, Krzyzanowska K, Wiesinger GF, Francesconi M, Wolzt M. Regular physical exercise normalizes elevated asymmetrical dimethylarginine concentrations in patients with type 1 diabetes mellitus. Wiener klinische Wochenschrift. 2005;117:816–820. doi: 10.1007/s00508-005-0476-y. [DOI] [PubMed] [Google Scholar]
- 19.Richter B, Niessner A, Penka M, Grdic M, Steiner S, Strasser B, Ziegler S, Zorn G, Maurer G, Simeon-Rudolf V, Wojta J, Huber K. Endurance training reduces circulating asymmetric dimethylarginine and myeloperoxidase levels in persons at risk of coronary events. Thrombosis and Haemostasis. 2005;94:1306–1311. doi: 10.1160/TH05-03-0158. [DOI] [PubMed] [Google Scholar]
- 20.Sydow K, Munzel T. ADMA and oxidative stress. Atherosclerosis. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 21.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 22.Michaels AD, Accad M, Ports TA, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106:1237–1242. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- 23.Nichols WW, Estrada JC, Braith RW, Owens K, Conti CR. Enhanced external counterpulsation treatment improves arterial wall properties and wave reflection characteristics in patients with refractory angina. Journal of the American College of Cardiology. 2006;48:1208–1214. doi: 10.1016/j.jacc.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 24.Pagonas N, Utz W, Schulz-Menger J, Busjahn A, Monti J, Thierfelder L, Dietz R, Klauss V, Gross M, Buschmann IR, Buschmann EE. Assessment of the effect of external counterpulsation on myocardial adaptive arteriogenesis by invasive functional measurements - design of the arteriogenesis network trial 2. International journal of cardiology. 2009 doi: 10.1016/j.ijcard.2009.05.050. In press. [DOI] [PubMed] [Google Scholar]
- 25.Buschmann EE, Utz W, Pagonas N, Schulz-Menger J, Busjahn A, Monti J, Maerz W, le Noble F, Thierfelder L, Dietz R, Klauss V, Gross M, Buschmann IR. Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.Net.-2 Trial) European journal of clinical investigation. 2009;39:866–875. doi: 10.1111/j.1365-2362.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa ET, Bottom KE, Xiao X, Kamm RD. Numerical simulation of enhanced external counterpulsation. Ann Biomed Eng. 2001;29:284–297. doi: 10.1114/1.1359448. [DOI] [PubMed] [Google Scholar]
- 27.Green D. Exercise training as vascular medicine: Direct impacts on the vasculature in humans. Exercise and Sport Sciences Reviews. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 28.Balady GJ, Chaitman B, Driscoll D, Foster C, Froelicher E, Gordon N, Pate R, Rippe J, Bazzarre T. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Circulation. 1998;97:2283–2293. doi: 10.1161/01.cir.97.22.2283. [DOI] [PubMed] [Google Scholar]
- 29.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100:793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 30.Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, Brunsvig A, Muller F, Forfang K, Froland SS, Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. Journal of the American College of Cardiology. 2001;37:485–491. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- 31.Postadzhiyan AS, Tzontcheva AV, Kehayov I, Finkov B. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and their association with clinical outcome, troponin T and C-reactive protein in patients with acute coronary syndromes. Clinical biochemistry. 2008;41:126–133. doi: 10.1016/j.clinbiochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 35.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. Journal of internal medicine. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Holmes DR., Jr Treatment options for angina pectoris and the future role of enhanced external counterpulsation. Clinical cardiology. 2002;25:II22–25. doi: 10.1002/clc.4960251407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols WW, editor. McDonald’s Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. 5. London: Edward Arnold; 2005. [Google Scholar]
- 39.Hashemi M, Hoseinbalam M, Khazaei M. Long-term effect of enhanced external counterpulsation on endothelial function in the patients with intractable angina. Heart Lung Circ. 2008;17:383–387. doi: 10.1016/j.hlc.2008.02.001. [DOI] [PubMed] [Google Scholar]


