Abstract
The ability of sigma1 receptors to interact with a huge range of drug structural classes coupled with its wide distribution in the body has contributed to it being implicated as a possible therapeutic target for a broad array of disorders ranging from substance abuse to depression to Alzheimer’s disease. Surprisingly, the reported affinity values for some sigma1 receptor ligands vary more than 50-fold. The potential of the sigma1 receptor as a pharmacotherapeutic target prompted us to develop an unambiguous assay system for measuring the affinity of ligands to the cloned human sigma1 receptor. In the course of characterizing this system and determining the true affinity values for almost three dozen compounds, it was discovered that some dopamine D4 receptor selective compounds bind sigma1 receptors with high affinity. A systematic analysis of haloperidol-like compounds revealed a clear structure-affinity relationship amongst clinically relevant butyrophenones. The antidepressant fluvoxamine, the drug of abuse methamphetamine, and the neurosteroid progesterone were amongst the many ligands whose interactions with sigma1 receptor were confirmed with our screening assay.
Keywords: antipsychotics, psychotomimetics, psychostimulants, N-methyl-D-aspartate receptor, opioid receptor, neuroprotection
1. Introduction
Sigma receptors are a mysterious family of proteins whose origin, properties and interactions with endogenous and exogenous ligands are steeped in controversy. Originally they were classified as members of the opiate receptor family to account for the psychotomimetic effects exhibited by racemic benzomorphans in the chronic spinal dog (Martin et al., 1976). However, the ineffectiveness of opioid receptor antagonists, naloxone and naltrexone, against behaviors induced by N-allylnormetazocine ((±)SKF-10047) later distinguished its identity as a non-opioid receptor (Su, 1982; Vaupel, 1983). The differences between the enantioselectivity of SKF-10047 and other benzomorphans for Sigma and opioid receptors further supported the unique classification for Sigma receptors (Zukin, 1982; Tam, 1983). Subsequently, controversy also arose over the identity of Sigma receptor sites versus PCP sites on NMDA receptors due to the similar affinities of several ligands for both sites (Quirion et al., 1987; Zukin et al., 1984), and the modulatory role sigma1 receptors have on NMDA receptors (Yamamoto et al., 1995) and glutamate release (Debonnel and de Montigny, 1996). When more selective compounds became available, PCP and sigma1 binding sites were established as belonging to distinct proteins (Tam 1983, Tam and Cook 1984). Similar types of misunderstandings for haloperidol sites on sigma1 receptors and dopamine D2-like receptors (i.e., D2, D3 and D4) have also arisen. This was due in part to the similar affinities of haloperidol and some other antipsychotic drugs for sigma1 and dopamine D2-like receptor sites in brain tissue (Tam and Cook, 1984) and the ability of sigma1 receptors to modulate dopamine release (Debonnel and de Montigny, 1996). Above all other reasons, the cause of the mistaken identity of sigma1 receptors as possibly belonging to other receptor families can be attributed to the uncanny ability of sigma1 receptors to bind to a remarkably large and diverse class of ligands with moderate to high affinity; this includes antipsychotics, antidepressants, neurosteroids, addictive psychotropics and other controlled substances (Walker et al., 1990; Hayashi and Su, 2004).
Two subtypes within the Sigma receptor family, sigma1 and sigma2, have been identified so far on the basis of their pharmacological properties (Bouchard et al., 1997, Leitner et al., 1994, Quirion et al., 1992). Only the sigma1 receptor has been cloned and this was first achieved using guinea pig liver as the tissue source (Hanner et al., 1996). Since then the sigma1 receptor has been cloned and characterized from human (Kekuda et al., 1996), mouse (Seth et al., 1997), and rat (Seth et al., 1998). The full length gene encodes for a protein containing 223 amino acids and whose sequence is entirely unique amongst other mammalian proteins. Its highest similarity is with a yeast C8-C7 sterol isomerase (30% identity and 64% homology), even though it has no detectable isomerase activity itself (Hanner et al., 1996). The proposed topology of the sigma1 receptor has been speculated on the basis of hydropathy analysis to consist of one putative transmembrane region (Hanner et al., 1996; Kekuda et al., 1996; Seth et al., 1997), although more recent antibody accessibility experiments suggest the existence of two transmembrane regions (Aydar et al., 2002). The presence of a transmembrane structure is consistent with sigma1 receptors being almost ubiquitously distributed within the membranes of various subcellular compartments including nuclear, Golgi, endoplasmic reticulum, mitochondrial, and plasmallemal, with the lowest levels in plasmallemal and the highest levels in mitochondrial and nuclear membranes (Falkenstein et al., 1998; Maurice et al., 1999; Morin-Surun et al., 1999; Lupardus et al., 2000; Hayashi and Su, 2001).
Sigma1 receptors are widely distributed throughout the body. In the periphery they are localized in the heart, kidneys, testes, ovaries, lungs, intestines, muscles, and liver (see Walker et al., 1990 for review). Ex vivo autoradiography and immunocytochemistry studies reveal that high densities of this receptor are also localized in the brain, especially in the brainstem, the cerebellum, and the prefrontal and parietal cortex, as well as in various limbic structures including the olfactory bulb, the hypothalamus, the hippocampus, and the thalamus, with lower levels in the striatum (Hashimoto et al., 1995; Alonso et al., 2000). Both neurons and glial cells are known to express this receptor (Alonso et al., 2000, Palacios et al., 2003).
The unparalleled ability of sigma1 receptors to interact with a huge range of drug structural classes and its wide distribution in the body has contributed to it being implicated as a possible therapeutic target for a broad array of disorders, including depression, psychosis, substance abuse, Alzheimer’s disease, cerebral stroke, and other traumatic brain injuries (see Maurice et al., 1999; Su and Hayashi 2003; Hayashi and Su 2004; Nguyen et al., 2005; Yagasaki et al., 2006; Meunier et al., 2006; Wang et al., 2007; Martin-Fardon et al., 2007). This potential of sigma1 receptors to serve as a pharmacotherapeutic target, or even as a side effect-mediating target, calls for a better understanding of the relationship between the sigma1 receptor and its ligands. In the past decades, reported affinity values for some sigma1 receptor ligands have varied more than 50-fold. Factors that may have contributed to this ambiguity include the utilization of non-selective radioligands, and tissues or cells containing many receptor types. [3H]-(+)-pentazocine was used as the radioligand in our studies, because it is the only readily-available high affinity radioligand suitable for a sigma1 binding assay. Since [3H]-(+)-pentazocine binds other receptors (e.g. opioid receptors) as well, we sought a cell line devoid of [3H]-(+)-pentazocine specific binding, which could serve as a null background for the stable expression of a cloned human sigma1 receptor. Using such a cell line, we then established a reliable system for screening sigma1 receptor ligands and measured true affinity values for almost three dozen compounds representing a wide range of structural classes of interest in the context of sigma1 receptors and compared the affinities with those reported previously. Several interesting findings arose during the course of this work and amongst them was the discovery that some heretofore dopamine D4 receptor selective compounds bind sigma1 receptors with high affinity. Further, a systematic study of all available haloperidol-like compounds indicated a clear structure-affinity relationship amongst clinically relevant butyrophenones.
2. Materials and Methods
2.1. Reagents
All drugs and reagents were purchased from Tocris (Ellisville, MO), Sigma-Aldrich Chemical Company and Fluka (St. Louis, MO). Cell culture supplies were purchased from Thermo Fisher Scientific (Logan UT). The [3H](+)-pentazocine (NET-1056, 36.6 Ci/mmol) was purchased from Dupont NEN.
2.2. Establishment of a stable cell line
MCF-7 cells (American Type Cell Culture, HTB-22) were cultured in 150 cm2 flasks (Sarstedt 83.1812) in Dulbecco’s Modified Eagle’s Medium (DMEM; Fisher Scientific SH30003.02) supplemented with 10% Bovine Calf Serum (Fisher Scientific 30072.03), 100 μg/ml non-essential amino acids (Hyclone SH3023801), 2mM L-glutamine (Sigma G8540), and 10 μg/L Bovine Insulin (Sigma-Aldrich 11070.73.8). Cells were kept in an incubator with 5% CO2 and 95% air and 95% humidity at 37 °C. The full length coding region of the cloned human sigma1 receptor DNA (Genbank accession no. BC004899) was obtained from American Type Culture Collection (MGC-3851) and its integrity was confirmed by sequencing. The full length sigma1 receptor was digested and subcloned into a pcDNA3.1 (Invitrogen, CA) vector, which was then transfected into MCF-7 cells using a calcium phosphate precipitation method (Invitrogen, CA). Individual clones were established over a period of weeks on the basis of G418 (2 mg/ml; InvivoGen, ant-gn-5) selection. The expression levels of the sigma1 receptor in individual clones were quantified by saturation isotherm binding using the sigma1 selective radioligand [3H](+)-pentazocine (Perkin-Elmer, specific activity 36.6-37.7 Ci/mmol).
2.3. Preparation of cell membranes
Cells were detached from culture flasks by 10 min incubation at 37 °C in Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (Fisher Scientific 55-031-PB) supplemented with 5 mM EDTA (Sigma Aldrich E6511). Cells were subsequently pelleted by centrifugation at 850×g with 4 volumes (v/v) of Earle’s balanced saline solution (EBSS; Fisher Scientific SH30014.03) for 10 minutes. The supernatant was decanted and the membrane pellet was lysed in ice cold buffer containing 5 mM Tris and 5 mM MgCl2 at pH 7.4. The membrane solution was then homogenized with 8 strokes of a Dounce glass-glass homogenizer and centrifuged at 35,000×g for 60 min. The supernatant was decanted and the membrane pellet was re-suspended in cold binding buffer (50 mM Tris, pH 7.4 at 4°C) by triturating with a polypropylene Pasteur pipette and re-centrifugation at 35,000×g for 60 min. The final membrane pellet was re-suspended by light homogenization (4 strokes) in warm binding buffer (50 mM Tris pH 8.0 at 37°C) immediately before use in the binding experiments.
2.4. Receptor binding assay and data analysis
All binding studies were carried out in a volume of 1 mL: each assay tube contained 50 μL of the non-isotopic drug or vehicle control, 100 μl of [3H](+)-pentazocine, 100 μL of membrane suspension, and 750 μL of 50 mM Tris binding buffer at pH 8.0. Protein concentration in each tube ranged from 5-30 μg/mL and was measured in triplicates using the bicinchonic acid (BCA) protein reagent (Pierce, IL) according to the manufacturer’s specification using a bovine serum albumin standard curve. All binding reactions were allowed 180 minutes to reach equilibrium at 37°C with moderate shaking and were terminated by rapid filtration through Whatman GF/C filters (Brandel FPD-205) pre-soaked in 0.3 % polyethyleneimine with the addition of three 2 mL volumes of ice-cold wash buffer (10 mM Tris, pH 8.0 at 2°C). Filter-bound radioactivity was quantified by liquid scintillation counting. Saturation isotherm binding assays were conducted utilizing eight concentrations of [3H]-(+)-pentazocine from 0.16 to 20 nM. For competition binding experiments, membranes were equilibrated with a fixed concentration of 1 nM of [3H](+)-pentazocine and thirteen different concentrations of the competing ligand over a three log unit range. Non-specific binding was defined by 5 μM haloperidol or 5 μM BD1063. The IC50 values were determined by non-linear regression curve fitting models with a 95% confidence interval using GraphPad’s Prism version 4.0. The statistical measures of curve fitting were the F-test, the run test, and a correlation coefficient (r2). The inhibition constant (Ki) values were calculated from IC50 values according to the Cheng-Prusoff equation: Ki = IC50/(1+[ligand]/KD (Cheng and Prusoff, 1973). Each value for each experiment was performed in triplicates and all experiments were repeated three times (n = 3).
3. Results
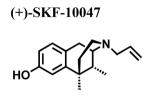
Since a variety of different methodologies for sigma1 receptor radioligand binding have been published, we systematically investigated the conditions required for optimization of equilibrium binding of the commercially-available, high affinity radioligand [3H ](+)-pentazocine. The parameters investigated included binding buffers in the range of 10-50 mM Tris at pH 7.2-8.0 at temperatures from 25-37°C and incubation times from 90-180 min (data not shown). Optimal conditions were determined to be 50 mM Tris buffer at pH 8.0 at 37°C and an incubation time of 180 minutes with continuous shaking. Under these conditions, the total binding of [3H ](+)-pentazocine to sigma1 receptors in crude membrane preparations was maximized and the nonspecific binding minimized. MCF-7 cells stably transfected with plasmid cDNA containing the full length cloned human sigma1 receptor (hS1R) exhibit dose-dependent and saturation specific binding for [3H ](+)-pentazocine, while untransfected MCF-7 cells do not (Fig. 1). The affinity and the receptor density were determined to be KD (S.D.) = 3.7 ± 0.87 nM and Bmax (S.D.) = 109 ± 23.7 pmoles/mg, respectively. Several ligands inhibited the binding of [3H](+)-pentazocine to membranes isolated from hS1R-transfected MCF-7 cells (hS1R-MCF-7) in a manner consistent with that of a sigma1 receptor (Table 1). For example, the sigma1 selective antagonist BD1063 bound hS1R-MCF-7 with high affinity and the agonists (±) SKF10047 displayed the classical enantiomeric-selectivity with (+)-SKF10047 binding with higher affinity than (−)-SKF10047 (Fig. 2). The rank order of Ki values for all the hS1R reference compounds and haloperidol was: 4-PPBP < haloperidol < BD1063 ≅ pentazocine < IPAB << PRE-084 << BMY14802 ≅ (+)-SKF10047 << (−)-SKF10047.
Fig. 1.
[3H]-(+)-pentazocine saturation isotherm binding to a clonal human MCF-7 cell line stably expressing the human sigma1 receptor. The average affinity (KD) and receptor density (Bmax) values (n = 3) are: 3.7 ± 0.87 nM and 109 ± 23.7 pmoles/mg protein, respectively. No specific [3H]-(+)-pentazocine binding was detected in untransfected MCF-7 cells, indicating the absence of drug-sensitive endogenous sigma1 or opioid receptors.
Table 1.
Nomenclature, chemical structures and comparison of affinity values generated in whole tissue and from a cloned human Sigma-1 receptor expressed in MCF-7 cells. Affinity values (Ki or KD, nM) are expressed a geometric means ± standard deviations. Average Ki values from available past studies using whole tissues are listed for comparison.A superscripted letter next to individual values indicates the reference, which is listed below.
| Compound Names and Structures | Ki values for the cloned hSigma-1 receptor |
Ki values measured in whole tissues |
Average Ki values from whole tissues |
|---|---|---|---|
| Sigma-1 Receptor Reference Compounds | |||
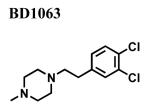
|
3.11 ± 2.40 | 9.15 i | 9 |
|
250 ± 79.1 | 19.4 a | 205 ± 339 |
| 48 d | |||
| 713 b | |||
| 41 l | |||
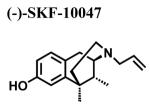
|
7132 ± 2551 | 84.9 a | 2247 ± 2104 |
| 1800 d | |||
| 5133 b | |||
| 1970 l | |||

|
3.70 ± 0.872 | 17 b | 8 ± 8 |
| 4.59 a | |||
| 1 l | |||
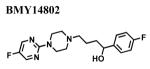
|
173 ± 58.2 | 306 b | 186 ± 170 |
| 66 m | |||

|
54.7 ± 23.9 | 9 n | 9 |

|
0.903 ± 0.397 | 1.14 h | 1.14 |

|
5.29 ± 0.978 | 2.57 c | 8 ± 7 |
| 13.1 j | |||
| Butyrophenone Antipsychotics and Metabolites | |||
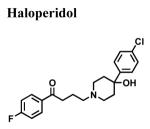
|
1.67 ± 0.801 | 3.12 a | 4 ± 3 |
| 0.2 l | |||
| 1.47 g | |||
| 4 d | |||
| 3 m | |||
| 10.2 b | |||
| 2.7 p | |||

|
128 ± 20.6 | 362 m | 344 ± 25 |
| 326 p | |||

|
1.54 ± 0.817 | 22 m | 14 ± 12 |
| 5.1 p | |||
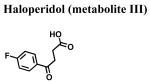
|
>10,000 | >10,000 m | >10,000 |

|
3.32 ± 0.0954 | 12 m | 12 |
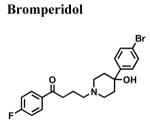
|
1.18 ± 0.355 | - | - |

|
1.28 ± 0.628 | - | - |

|
2241 ± 864 | - | - |

|
241 ± 64.1 | - | - |

|
1054 ± 579 | 1090 d | 1090 |

|
147 ± 39.5 | - | - |
| Antipsychotics/Antidepressants | |||

|
159.0 ± 57.4 | 144 d | 736 ± 733 |
| 508 o | |||
| 1,555 m | |||
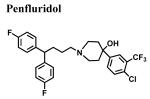
|
53.8 ± 25.2 | - | - |

|
13.9 ± 4.68 | 17 d | 63 ± 46 |
| 62 o | |||
| 109 m | |||

|
>10,000 | 11400 d | >10,000 |
| >10,000 m | |||

|
7.09 ± 3.18 | 36 e | 36 |
| D4 Dopamine Receptor Selective Ligands | |||

|
63.0 ± 22.7 | - | - |

|
4.80 ± 0.919 | - | - |
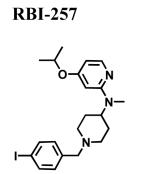
|
2.48 ± 0.580 | - | - |

|
5161 ± 2670 | - | - |
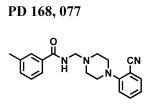
|
2612 ± 269 | - | - |
| Drugs of Abuse | |||

|
5248 ± 1044 | 2160 f | 2160 |
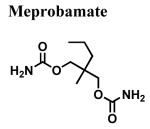
|
>100,000 | - | - |
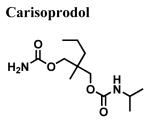
|
>100,000 | - | - |
| Endogenous Neurosteroids | |||

|
468 ± 139 | 268 g | 235 ± 143 |
| 24.6 a | |||
| 338 l | |||
| 338 q | |||

|
>10,000 | - | - |
Fig. 2.
Displacement of [3H]-(+)-pentazocine binding by a sigma1 selective antagonist (BD1063) and by stereoisomers of a prototypical benzomorphan agonist (+)-SFK10,047 and (−)-SKF10,077. Binding studies utilized cell homogenates derived from membranes of MCF-7 cells stably expressing the human sigma1 receptor. The affinity values (Ki) are listed in Table 1.
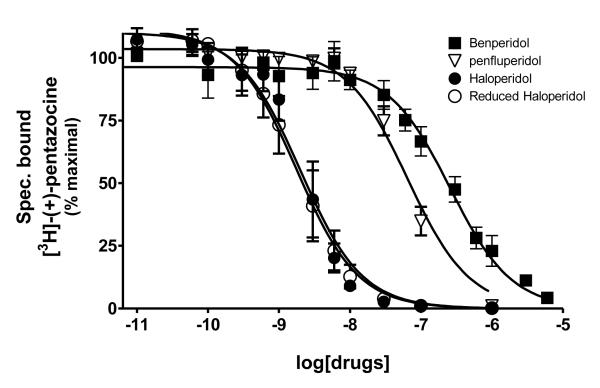
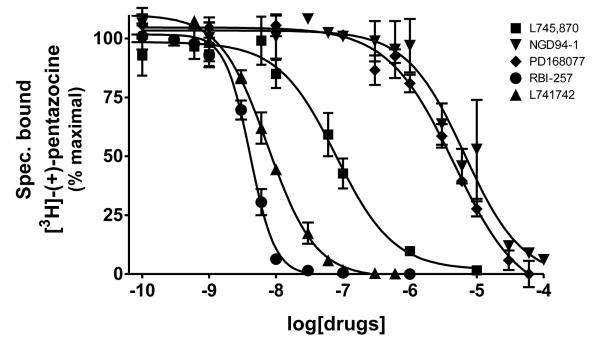
The classical sigma1 receptor antagonists haloperidol and its reduced metabolite (i.e., metabolite II) both have high, and nearly indistinguishable, affinities for hS1R-MCF-7, while the haloperidol derivatives penfluridol and benperidol displayed much lower affinities (Fig. 3). The rank order of Ki values for haloperidol, its primary metabolites, clinically-relevant butyrophenones, as well as other commercially available butyrophenones was: bromperidol ≅ chloroperidol ≅ haloperidol metabolite II ≅ haloperidol < trifluperidol < haloperidol metabolite I ≅ 3′fluorobenzylspiperone < benperidol < spiperone < droperidol << haloperidol metabolite III (no detectable affinity). Since all these butyrophenones bind non-selectively and with high affinity to dopamine D2-like receptors (i.e., dopamine D2, D3 and D4 receptors), a panel of compounds that bind selectively and with high affinity to dopamine D4 receptors were screened for hS1R-MCF-7 binding activity. Both RBI-257 and L741,742 had high affinity for hS1R-MCF-7 in the low nanomolar range, while L745,870 had a mid nanomolar affinity (Fig. 4, Table 1). However, PD168,007 and NGD 94-1 had much lower affinities that were in the micromolar range (Table 1).
Fig. 3.
Displacement of [3H]-(+)-pentazocine binding by butyrophenones and reduced haloperidol. Butyrophenone class compounds bind to the sigma1 receptor with varying degrees of affinity. Reduced haloperidol exhibits similar binding as haloperidol. The affinity values (Ki) are listed in Table 1.
Fig. 4.
Displacement of [3H]-(+)-pentazocine binding by dopamine D4 receptor selective compounds. Several dopamine D4 receptor selective drugs also bind to sigma1 receptors with a range of affinities. The rank order for these drug affinities is: RBI-257 >> L745,870 >> PD168,077 > NGD94-1. The affinity values (Ki) are listed in Table 1.
Other compounds were also screened for binding to the cloned sigma1 receptor including typical antipsychotics such as pimozide, penfluridol and fluphenazine, and the atypical antipsychotic clozapine (Table 1). Of these, fluphenazine had the highest affinity, while clozapine had no detectable level of binding. The antidepressant fluvoxamine had moderately high affinity for hS1R-MCF-7 comparable to that of fluphenazine. Methamphetamine was the only one of three drugs of abuse tested that displayed detectable levels of binding and its affinity was in the low micromolar range (Table 1). The neurosteroid progesterone had a submicromolar affinity, while ß-estradiol exhibited no detectable binding (Table 1).
Comparison of the affinity values determined here for hS1R-MCF-7 and in membranes from whole tissues reported in the literature revealed that the composite affinity values, determined by averaging values from available whole tissues studies, were often in agreement with those from hS1R-MCF-7 even though individual values from different whole tissue studies sometimes varied over 50-fold (Table 1). For example, of the seven values reported in the literature for haloperidol binding to sigma1 receptors in whole tissue with a range of values from 0.2 to 10.2 nM the averaged value of 4 ± 3 nM was in agreement with the value of 1.67 ± 0.801 nM determined here for hS1R-MCF-7 (Table 1). Similar results are evident for the enantiomers of SKF10047. The (+)-SKF10047 had a whole tissue affinity range of 19.4 nM to 713 nM and an averaged value of 205 ± 339 nM, which is comparable to the hS1R-MCF-7 value of 250 ± 79.1 nM. The (−)-SKF10047 had a whole tissue affinity range of 84.9 nM to 5133 nM and an averaged value of 2247 ± 2104 nM that is similar to the hS1R-MCF-7 value 7132 ± 2551 nM. The largest differences between values reported in whole tissue and in hS1R-MCF-7 were for the S1R-selective agonist PRE-084, the antidepressant fluvoxamine, the antipsychotics fluphenazine and pimozide, and haloperidol metabolite II: averaged values for all these drugs determined in whole tissue differed from those in hS1R-MCF-7 by over 4.5-fold (Table 1).
4. Discussion
The majority of studies investigating the affinity of ligands for the sigma1 receptor have utilized membranes purified from whole tissues (see reference in Table 1), which is still a common practice (Weigle and Wunsch, 2007; Xu et al., 2007; Chen et al., 2007), despite the cloning of the first sigma1 receptor over a decade ago (Hanner et al., 1996). Further, most studies of cloned sigma1 receptors have utilized cell lines containing endogenous sigma1 receptors as hosts for over-expression of the cloned receptor (Kekuda et al., 1996; Seth et al., 1997; Ganapathy 1999; Mei and Pasternak 2001). This may be due in part to the almost ubiquitous nature of sigma1 receptors. Remarkably, the human breast adenocarcinoma MCF-7 cell line is the only line specifically reported as having little or no detectable binding for [3H]-(+)-pentazocine, a typical marker for sigma1 receptors (Vilner et al., 1995). Curiously, the mRNA for the sigma1 receptor has been reported to be present (Aydar et al. 2005) and absent (Seth et al., 1998) in MCF-7 cells. Immunocytochemistry of MCF-7 cells using an antibody made against a purified human sigma1 receptor (Jbilo et al., 1997) revealed immunofluorescence only in MCF-7 cells transfected with human sigma1 receptors, but not untransfected cells (Seth et al., 2001). Western blot analyses of proteins isolated from MCF-7 cells with antibodies made against peptide fragments of the human receptor revealed protein bands with no molecular weight reported (Wang et al., 2004), ~15-18 kDa (Mukherjee et al., 2005) or 25 kDa (Aydar et al., 2005). The former two studies utilized a commercially-available antibody that recognizes a peptide sequence between amino acids 50-100 (personal communication, Kent Williams, Santa Cruz Biotechnologies, Inc.). According to the company’s data sheet for Cat. No. sc-16230, the antibody binds a band close to ~28-30 kDa that in any case higher than the expected maximum 25 kDa size of the native protein (see http://www.santacruzbiotechnology.com/picts.php?pict_id=32364). In the latter study, rabbits were immunized simultaneously with two different peptides (amino acids 37-51 and 137-158) and the resulting polyclonal antibodies were immunopurified against the immunizing peptides. In all ten cell lines tested, these antibodies stained a protein band whose molecular weight is determined to be that expected for the human sigma1 receptor (~25 kDa) and whose intensity is reduced after treating MCF-7 cells with sigma1 receptor pSilencer siRNA (Aydar et al., 2005). Although it does not fully account for the size discrepancies, it is noteworthy that an alternatively spliced variant of the sigma1 receptor missing the 96 bp portion of the receptor corresponding to exon 3 (amino acids 119-149) is insensitive to drugs like pentazocine because this region harbors the binding site for sigma1 receptor ligands (Ganapathy et al., 1999). Regardless of the reason for these discrepancies, our finding that untransfected MCF-7 cells have no detectable specific binding for the sigma1 receptor radioligand [3H]-(+)-pentazocine is consistent with previous reports (Vilner et al., 1995; Seth et al., 1998). This absence of [3H]-(+)-pentazocine specific binding allowed us to exploit MCF-7 cells as a null background for the expression of a cloned sigma1 receptor and provided an assay system for the unambiguous evaluation of ligand affinity values.
Stable expression of the full-length, cloned sigma1 receptor in MCF-7 cells results in high affinity [3H]-(+)-pentazocine binding within the range expected for a cloned sigma1 receptor (Mei and Pasternak, 2001) and for sigma1 receptors in whole tissues (see references in Table 1). The human sigma1 receptor stably expressed in the MCF-7 cell line also displayed a pharmacological profile consistent with that of a sigma1 receptor. This includes the classical enantioselective higher affinity for (+)-SKF10047 versus (−)-SKF10047, as well as high affinity binding for the sigma1 antagonists haloperidol and BD1063, the sigma1 selective agonists PRE-084 and IPAB, and the sigma1 ligands 4-PPBP and BMY14802. Further, the cloned human receptor bind both haloperidol and reduced haloperidol (metabolite II) with nearly identical affinities and has reduced but still detectable affinity for haloperidol metabolite I, which is consistent with a sigma1 receptor profile and clearly distinguishes it from that of other receptors that bind haloperidol with high affinity such as rat dopamine D2-like receptors (Bowen et al., 1990). This cumulative pharmacological profile establishes that our radioligand binding assay conditions, including the use of MCF-7 cells as a null background for expressed cloned sigma1 receptors, allow for the unambiguous assignment of affinity values for ligands binding to the human sigma1 receptor. The true affinity values were measured for thirty-four compounds representing a wide range of structural classes of interest in the context of sigma1 receptors and compared to the affinities with those reported previously; in the past decades, reported affinity values for some sigma1 receptor ligands have varied more than 50-fold.
A systematic structure-affinity study was conducted for all clinically-relevant haloperidol-like ligands, with an emphasis on those belonging to the butyrophenone structural class or that are butyrophenone derivatives, because haloperidol is a classical sigma1 receptor antagonist and a subject of many clinical investigations. Amongst this group, a clear structure-affinity relationship emerged: high affinity binding to the sigma1 receptor required the presence of both a 4-linked halogenated phenyl and an electronegative moiety at position 4 along the butyl chain (Table 1). Haloperidol, chlorohaloperidol, bromperidol, trifluperidol, and reduced haloperidol each have a 4-linked halogenated phenyl substructure in combination with an electronegative moiety at the 1-position along the butyl chain (either a keto (C=O) or a hydroxyl (-OH)) and high affinity for the sigma1 receptor. In contrast, the butyrophenones spiperone, 3′-fluorbenzylspiperone, benperidol, and droperidol each have an electronegative keto moiety (C=O) along the butyl chain, but they do not possess a 4-linked phenyl substructure and only have low affinity for the sigma1 receptor. Both penfluridol and haloperidol metabolite I have a 4-linked halogenated phenyl but lack either an electronegative moiety along the butyl chain or the butyl chain all together, resulting in moderate affinity for the sigma1 receptor. The non-butyrophenone compounds, BMY14802 and pimozide, have some sub-structural resemblance and moderate affinities as well. However, the very high affinity binding of 4-PPBP to the sigma1 receptor indicates that while a 4-linked halogenated phenyl and a 1-electronegative along the butyl chain are common structural features of high affinity butyrophenones, neither of these features are absolutely required for the high affinity interactions. Rather an appropriately spaced C-N(R)-X-Ph motif appears to be most critical (Glennon, 2005).
Along with the notable differences in binding affinities among butyrophenone antipsychotics, the structure-affinity relationships point to the lack of association between antipsychotic treatments and the sigma1 receptor. Droperidol and haloperidol, for example, are both potent antipsychotics but differ greatly in their binding affinities for the sigma1 receptor, suggesting that the antipsychotic effects are not mediated by the sigma1 receptor. In addition, haloperidol and reduced haloperidol (a haloperidol metabolite) bind to the sigma1 receptor with an almost identical affinity, yet haloperidol is a highly potent neuroleptic while reduced haloperidol has no antipsychotic activity. Furthermore, non-butyrophenone antipsychotics such as clozapine have no detectable affinity for the sigma1 receptor. This demonstrates that sigma1 receptors do not mediate the antipsychotic activity of butyrophenones or other antipsychotic drugs, even though certain antipsychotic drugs, such as fluphenazine, coincidentally bind with relatively high affinity to sigma1 receptors. The lack of a role for the sigma1 receptor in treating psychosis is further supported by clinical studies with selective sigma1 receptor antagonists, which have generally failed to show any efficacy for the treatment of the acutely psychotic symptoms of schizophrenia; however, it is still not clear whether negative symptoms might improve (for a review see Volz and Stoll 2004). Of interest in this regard is the idea that antidepressants, including selective serotonin reuptake inhibitors (SSRI) like fluvoxamine, may be useful add-on treatments for the negative symptoms of schizophrenia (for a review see Rummel et al., 2005; Moller, 2005). Incidentally, the relatively high affinity of fluvoxamine and selected other antidepressants has led some to suggest that sigma1 receptors may play a role in the treatment of depression (Narita et al., 1996; Yagasaki et al., 2006; Wang et al., 2007; Dhir and Kulkarni, 2007) or obsessive-compulsive disorder (Egashira et al., 2007). The affinity we determined for the binding of fluvoxamine to the cloned human sigma1 receptor is about five-fold higher than that reported in whole tissue (Narita et al., 1996).
The affinity of some typical dopamine D4 receptor selective compounds for the sigma1 receptor was investigated, because of the historical tendency for drugs that bind dopamine D2 and D3 receptor subtypes to also bind to sigma1 receptors, and because an initial report hinted that a drug developed to be selective for the dopamine D4 receptor subtype might also bind to Sigma receptor subtypes (Patel et al., 1997). Here we report for the first time that some of the most commonly used dopamine D4 receptor selective antagonists, L745,870, L741,741 and RBI-257, have nanomolar affinities for the cloned sigma1 receptor, with the latter two having very high affinities that are within 10-fold of that reported for D4 receptors (Kulagowski et al., 1996; Rowley et al., 1996; Kula et al., 1999). In contrast, the dopamine D4 receptor selective agonist PD168,077 (Glase et al., 1997) and antagonist NGD 94-1 (Tallman et al., 1997) have micromolar affinities for the sigma1 receptor. The ability of some, but not other, dopamine D4 receptor selective compounds to bind sigma1 receptors with high affinity may be relevant to the pharmacological dissection of memory responses, because sigma1 receptors play a role in memory (Maurice and Lockhart, 1997; Meunier et al., 2004; Phan et al., 2003; Hiramatsu and Hoshino, 2005; Hashimoto et al., 2007) and dopamine D4 receptors are involved in encoding fear extinction (Floresco and Magyar, 2006; Pfeiffer and Fendt, 2006). Although it does not account for the inability to be fully displaced by high concentrations of haloperidol or L745,870, it is possible that the lack of autoradiographic regional selectivity and unsaturability of [125I]RBI-257, and the inconsistent dopamine D4 receptor selective pharmacological profile of its des-iodo-derivative [3H]PNU-101,958 in rat brain (Kula et al., 1999) may in part be due to the high affinity of such compounds for the omnipresent sigma1 receptor.
Sigma1 receptors are thought to be a common link for the effects of drugs of abuse, including methamphetamine, and the low micromolar affinity we report for methamphetamine binding to the cloned human sigma1 receptor is consistent with that reported in whole P2 fractioned rat brain membranes (Nguyen et al., 2005). Other reports have also implicated sigma1 receptor antagonists in attenuating methamphetamine-induced behavioral sensitization (Takahashi et al., 2000; Ujike et al., 1992) and up-regulation of [3H](+)-pentazocine binding in the frontal cortex, cerebellum, and substantia nigra of rats exposed to methamphetamine (Ithzak, 1993). Therapeutic dosing of methamphetamine for use as a decongestant results in a peak blood concentration of around 0.02 mg/L (Logan, 2002), which corresponds to 134 nM, and is a concentration that would have negligible effects on the sigma1 receptor. However, toxic blood levels of methamphetamine range from approximately 0.2 to 5.0 μg/ml, and fatal levels of methamphetamine in the blood exceed 10 μg/ml (Inoue et al., 2006). The molar equivalents of these levels are approximately 1.3 μM to 34 μM and 67 μM, respectively. At these concentrations, there would be significant binding to the sigma1 receptor suggesting that the sigma1 receptor could possibly play a role in methamphetamine toxicity and fatality.
While the true endogenous ligand for sigma1 receptors has yet to be discovered (Maurice et al., 1999), sigma1 receptors do contain a sterol-sensitive binding site. Progesterone has been proposed as an endogenous ligand for the sigma1 receptor because its affinity is the highest amongst neurosteroids with a reported whole tissue Ki = 268-376 nM using either [3H](+)-SKF-10,047 or [3H]haloperidol as the radioligand (Su et al., 1988). The plasma levels of progesterone in women typically range from approximately 2-30 nM, depending upon the phase of the menstrual cycle, to a high of 450 nM during late-stage pregnancy (Johansson, 1969; Massai et al., 1999). This has cast some doubt on the designation of progesterone as an endogenous ligand (for a review see Bermack et al., 2005), because the level of occupancy would typically be relatively low and even at the highest plasma levels (450 nM), the occupancy would be approximately 50% based on our affinity values (Ki = 468 nM) for the cloned sigma1 receptor. Further, the levels of progesterone are much lower (about 1 nM) and do not fluctuate significantly in healthy men and prepubescent children (Trotter et al., 2001). Perhaps future research will reveal a better candidate ligand more suited for the role of the endogenous ligand of this enigmatic receptor.
Acknowledgements
The authors wish to thank Guillermo Tays for assisting with some of the assays in the later phases of this work, and Ethan Poteet for critical reading of the manuscript. Completion of this work was in partial fulfillment of an undergraduate honor’s college thesis by I.T.L. Special thanks goes to Dr. Robert McMahon who served as the honor’s college faculty mentor component for the thesis. This work was support in part by grant R01 MH063162 and funds G67710 both awarded to J.A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Debonnel G. The role of sigma receptors in depression. J. Pharmacol. Sci. 2005;97:317–336. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–477. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Bowen WD, Moses EL, Tolentino PJ, Walker JM. Metabolites of haloperidol display preferential activity at sigma receptors compared to dopamine D-2 receptors. Eur. J. Pharmacol. 1990;177:111–118. doi: 10.1016/0014-2999(90)90260-d. [DOI] [PubMed] [Google Scholar]
- Brown C, Fezoui M, Selig WM, Schwartz CE, Ellis JL. Antitussive activity of sigma1 receptor agonists in the guinea-pig. Br. J. Pharmacol. 2004;141:233–240. doi: 10.1038/sj.bjp.0705605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hajipour AR, Sievert MK, Arbabian M, Ruoho AE. Characterization of the cocaine binding site on the sigma1 receptor. Biochemistry. 2007;46:3532–3542. doi: 10.1021/bi061727o. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Debonnel G, de Montigny C. Modulation of NMDA and dopaminergic neurotransmissions by sigma ligands: possible implications for the treatment of psychiatric disorders. Life Sci. 1996;58:721–734. doi: 10.1016/0024-3205(95)02248-1. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Involvement of sigma1 receptor modulation in the antidepressant action of venlafaxine. Neurosci. Lett. 2007;420:204–208. doi: 10.1016/j.neulet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Egashira N, Harada S, Okuno R, Matsushita M, Nishimura R, Mishima K, Iwasaki K, Orito K, Fujiwara M. Involvement of the sigma1 receptor in inhibiting activity of fluvoxamine on marble-burying behavior: comparison with paroxetine. Eur. J. Pharmacol. 2007;563:149–154. doi: 10.1016/j.ejphar.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Schmieding K, Lange A, Meyer C, Gerdes D, Welsch U, Wehling M. Localization of a putative progesterone membrane binding protein in porcine hepatocytes. Cell Mol. Biol. 1998;44:571–578. [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J. Pharmacol. Exp. Ther. 1999;289:251–260. [PubMed] [Google Scholar]
- Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD. Substituted [(4-phenylpiperazinyl)-methyl]benzamides: selective dopamine D4 agonists. J. Med. Chem. 1997;40:1771–1772. doi: 10.1021/jm970021c. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Pharmacophore identification for sigma1 (σ1) receptor binding: application of the “deconstruction-reconstruction-elaboration” approach. Mini Rev. Med. Chem. 2005;5:927–940. doi: 10.2174/138955705774329519. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, London ED. Further characterization of [3H]ifenprodil binding to sigma receptors in rat brain. Eur. J. Pharmacol. 1993;236:159–163. doi: 10.1016/0014-2999(93)90241-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Scheffel U, London ED. In vivo labeling of sigma receptors in mouse brain with [3H]4-phenyl-1-(4-phenylbutyl)piperidine. Synapse. 1995;20:85–90. doi: 10.1002/syn.890200112. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma1 receptors. Neuropsychopharmacology. 2007;32:514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. sigma1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Hoshino T. Improvement of memory impairment by (+)- and (−)- pentazocine via sigma, but not kappa opioid receptors. Brain. Res. 2005;1057:72–80. doi: 10.1016/j.brainres.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Inoue H, Ikeda N, Kudo K, Ishida T, Terada M, Matoba R. Methamphetamine-related sudden death with a concentration which was of a ‘toxic level’. Leg. Med. (Tokyo) 2006;8:150–155. doi: 10.1016/j.legalmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Repeated methamphetamine-treatment alters brain sigma receptors. Eur. J. Pharmacol. 1993;230:243–244. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Vidal H, Paul R, De Nys N, Bensaid M, Silve S, Carayon P, Davi D, Galiegue S, Bourrie B, Guillemot JC, Ferrara P, Loison G, Maffrand JP, Le Fur G, Casellas P. Purification and characterization of the human SR 31747A-binding protein. A nuclear membrane protein related to yeast sterol isomerase. J. Biol. Chem. 1997;272:27107–27115. doi: 10.1074/jbc.272.43.27107. [DOI] [PubMed] [Google Scholar]
- Johansson ED. Plasma levels of progesterone in pregnancy measured by a rapid competitive protein binding technique. Acta. Endocrinol. 1969;61:607–617. doi: 10.1530/acta.0.0610607. [DOI] [PubMed] [Google Scholar]
- John CS, Bowen WD, Fisher SJ, Lim BB, Geyer BC, Vilner BJ, Wahl RL. Synthesis, in vitro pharmacologic characterization, and preclinical evaluation of N-[2-(1′-piperidinyl)ethyl]-3-[125I]iodo-4-methoxybenzamide (P[125I]MBA) for imaging breast cancer. Nucl. Med. Biol. 1999;26:377–382. doi: 10.1016/s0969-8051(98)00104-8. [DOI] [PubMed] [Google Scholar]
- John CS, Bowen WD, Saga T, Kinuya S, Vilner BJ, Baumgold J, Paik CH, Reba RC, Neumann RD, Varma VM. A malignant melanoma imaging agent: synthesis, characterization, in vitro binding and biodistribution of iodine-125-(2-piperidinylaminoethyl)4-iodobenzamide. J. Nucl. Med. 1993;34:2169–2175. [PubMed] [Google Scholar]
- Karasawa J, Takahashi S, Horikomi K. Binding properties of [3H]MS-377, a novel sigma receptor ligand, to rat brain membranes. Eur. J. Pharmacol. 2000;400:51–57. doi: 10.1016/s0014-2999(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem. Biophys. Res. Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Kula NS, Tarazi FI, Baldessarini RJ, Xu L, Bakthavachalam V, Pounds S, True CD. Neuropharmacological assessment of potential dopamine D4 receptor-selective radioligands. Eur. J. Pharmacol. 1999;367:139–142. doi: 10.1016/s0014-2999(98)00980-7. [DOI] [PubMed] [Google Scholar]
- Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Patel S, Ragan CI, Leeson PD. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J. Med. Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- Largent BL, Gundlach AL, Snyder SH. Psychotomimetic opiate receptors labeled and visualized with (+)-[3H]3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Proc. Natl. Acad. Sci. U.S.A. 1984;81:4983–4987. doi: 10.1073/pnas.81.15.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner ML, Hohmann AG, Patrick SL, Walker JM. Regional variation in the ratio of sigma 1 to sigma 2 binding in rat brain. Eur. J. Pharmacol. 1994;259:65–69. doi: 10.1016/0014-2999(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Logan BK. Methamphetamine - Effects on Human Performance and Behavior. Forens. Sci. Rev. 2002;14:133–151. [PubMed] [Google Scholar]
- Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J. Physiol. 2000;526:527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential Effects of sigma(1) Receptor Blockade on Self-Administration and Conditioned Reinstatement Motivated by Cocaine vs Natural Reward. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301323. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Massai R, Miranda P, Valdes P, Lavin P, Zepeda A, Casado ME, Silva MA, Fetis G, Bravo C, Chandia O, Peralta O, Croxatto HB, Diaz S. Preregistration study on the safety and contraceptive efficacy of a progesterone-releasing vaginal ring in Chilean nursing women. Contraception. 1999;60:9–14. doi: 10.1016/s0010-7824(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Pouw B. Correlation between neuroleptic binding to sigma(1) and sigma(2) receptors and acute dystonic reactions. Eur. J. Pharmacol. 2000;401:155–60. doi: 10.1016/s0014-2999(00)00430-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (σ1) receptor: pharmacological evidence and therapeutic opportunities. Jpn. J. Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su TP. Sigma1 and sigma2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem. Pharmacol. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Meunier J, Gue M, Recasens M, Maurice T. Attenuation by a sigma1 (σ1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br. J. Pharmacol. 2004;142:689–700. doi: 10.1038/sj.bjp.0705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius FF, Burrows GG, Hanner M, Schmid E, Striessnig J, Glossmann H. Identification of a 27-kDa high affinity phenylalkylamine-binding polypeptide as the sigma 1 binding site by photoaffinity labeling and ligand-directed antibodies. Mol. Pharmacol. 1993;44:966–971. [PubMed] [Google Scholar]
- Moller HJ. Antidepressive effects of traditional and second generation antipsychotics: a review of the clinical data. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255:83–93. doi: 10.1007/s00406-005-0580-z. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Collin T, Denavit-Saubie M, Baulieu EE, Monnett FP. Intracellular s1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc. Natl. Acad. Sci. U.S.A. 1999;14:8196–8199. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Prasad TK, Rao NM, Banerjee R. Haloperidol-associated stealth liposomes: a potent carrier for delivering genes to human breast cancer cells. J. Biol. Chem. 2005;280:15619–15627. doi: 10.1074/jbc.M409723200. [DOI] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Vela JM, Molina-Holgado E, Guitart X, Ovalle S, Zamanillo D. Immunohistochemical localization of the sigma1-receptor in oligodendrocytes in the rat central nervous system. Brain Res. 2003;961:92–99. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, Lynch JJ, Ragan CI. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J. Pharmacol. Exp. Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Phan VL, Urani A, Sandillon F, Privat A, Maurice T. Preserved sigma1 (σ1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol. Aging. 2003;24:865–881. doi: 10.1016/s0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Quirion R, Chicheportiche R. Classification and nomenclature of phencyclidine and sigma receptor sites. Trends Pharmacol. Sci. 1987;10:444–446. [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Cocaine-sensitive sigma-receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology. 1995;136:924–932. doi: 10.1210/endo.136.3.7867601. [DOI] [PubMed] [Google Scholar]
- Rowley M, Broughton HB, Collins I, Baker R, Emms F, Marwood R, Patel S, Patel S, Ragan CI, Freedman SB, Leeson PD. 5-(4-Chlorophenyl)-4-methyl-3-(1-(2-phenylethyl)piperidin-4-yl)isoxazole: a potent, selective antagonist at human cloned dopamine D4 receptors. J. Med. Chem. 1996;39:1943–1945. doi: 10.1021/jm960072u. [DOI] [PubMed] [Google Scholar]
- Rummel C, Kissling W, Leucht S. Antidepressants as add-on treatment to antipsychotics for people with schizophrenia and pronounced negative symptoms: a systematic review of randomized trials. Schizophr. Res. 2005;80:85–97. doi: 10.1016/j.schres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Seth P, Ganapathy ME, Conway SJ, Bridges CD, Smith SB, Casellas P, Ganapathy V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta. 2001;1540:59–67. doi: 10.1016/s0167-4889(01)00117-3. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid Binding at Sigma Receptors Suggests a Link Between Endocrine, Nervous, and Immune Systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma1 receptors: towards a hypothesis that sigma1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci. Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Primus RJ, Brodbeck R, Cornfield L, Meade R, Woodruff K, Ross P, Thurkauf A, Gallager DW. I. NGD 94-1: identification of a novel, high-affinity antagonist at the human dopamine D4 receptor. J. Pharmacol. Exp. Ther. 1997;282:1011–1019. [PubMed] [Google Scholar]
- Tam SW. Naloxone-inaccessible sigma receptor in rat central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1983;80:6703–6707. doi: 10.1073/pnas.80.21.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SW, Cook L. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H] SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter A, Muck K, Grill HJ, Schirmer U, Hannekum A, Lang D. Gender-related plasma levels of progesterone, interleukin-8 and interleukin-10 during and after cardiopulmonary bypass in infants and children. Crit. Care. 2001;5:343–348. doi: 10.1186/cc1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Okumura K, Zushi Y, Akiyama K, Otsuki S. Persistent supersensitivity of sigma receptors develops during repeated methamphetamine treatment. Eur. J. Pharmacol. 1992;211:323–328. doi: 10.1016/0014-2999(92)90388-k. [DOI] [PubMed] [Google Scholar]
- Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. Sigma1 and sigma2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- Volz HP, Stoll KD. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S214–220. doi: 10.1055/s-2004-832680. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol. Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Wang B, Rouzier R, Albarracin CT, Sahin A, Wagner P, Yang Y, Smith TL, Meric-Bernstam F, Aldaz C. Marcelo, Hortobagyi GN, Pusztai L. Expression of sigma 1 receptor in human breast cancer. Breast Cancer Res. Treat. 2004;87:205–214. doi: 10.1007/s10549-004-6590-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur. Neuropsychopharmacol. 2007 doi: 10.1016/j.euroneuro.2007.02.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigl M, Wunsch B. Synthesis of bridged piperazines with sigma receptor affinity. Eur. J. Med. Chem. 2007 doi: 10.1016/j.ejmech.2007.02.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Xu R, Lever JR, Lever SZ. Synthesis and in vitro evaluation of tetrahydroisoquinolinyl benzamides as ligands for sigma receptors. Bioorg. Med. Chem. Lett. 2007;17:2594–2597. doi: 10.1016/j.bmcl.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto T, Sagi N, Klenerova V, Goji K, Kawai N, Baba A, Takamori E, Moroji T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J. Neurosci. 1995;15:731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin SR. Differing stereospecificities distinguish opiate receptor subtypes. Life Sci. 1982;31:1307–1310. doi: 10.1016/0024-3205(82)90368-x. [DOI] [PubMed] [Google Scholar]
- Zukin SR, Brady KT, Slifer BL, Balster RL. Behavioral and biochemical stereoselectivity of sigma opiate/PCP receptors. Brain Res. 1984;294:174–177. doi: 10.1016/0006-8993(84)91326-x. [DOI] [PubMed] [Google Scholar]