Abstract
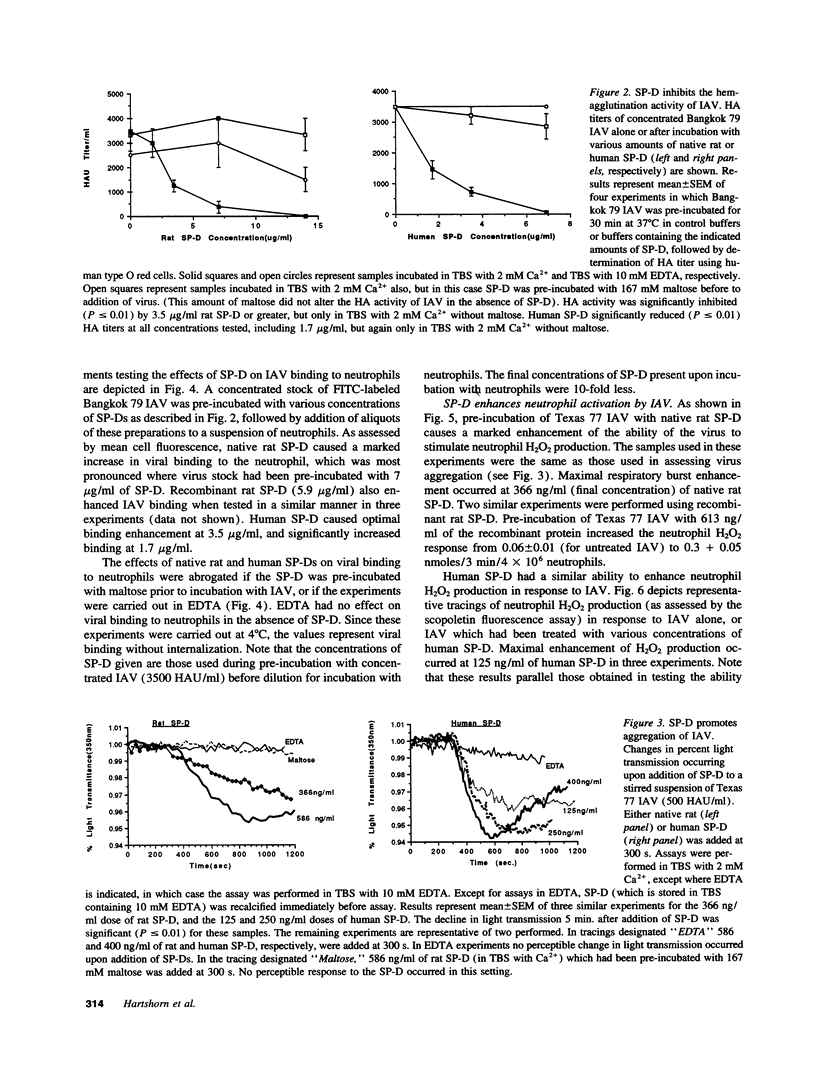
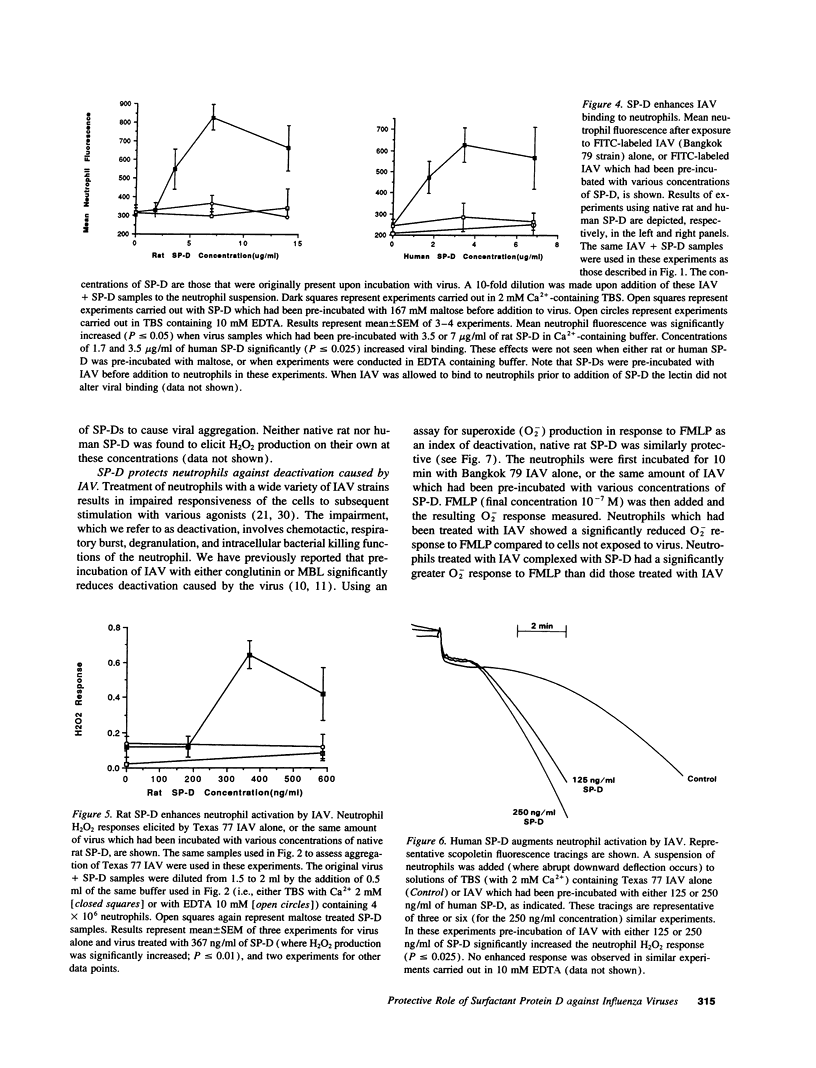
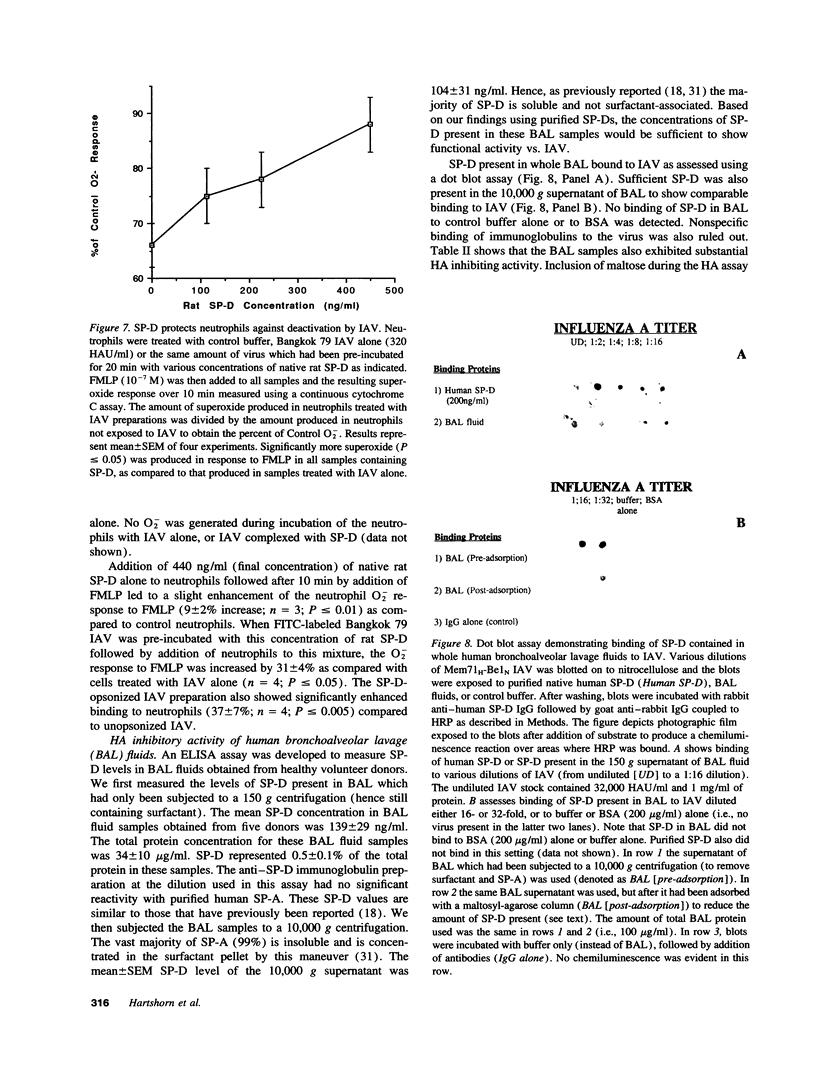
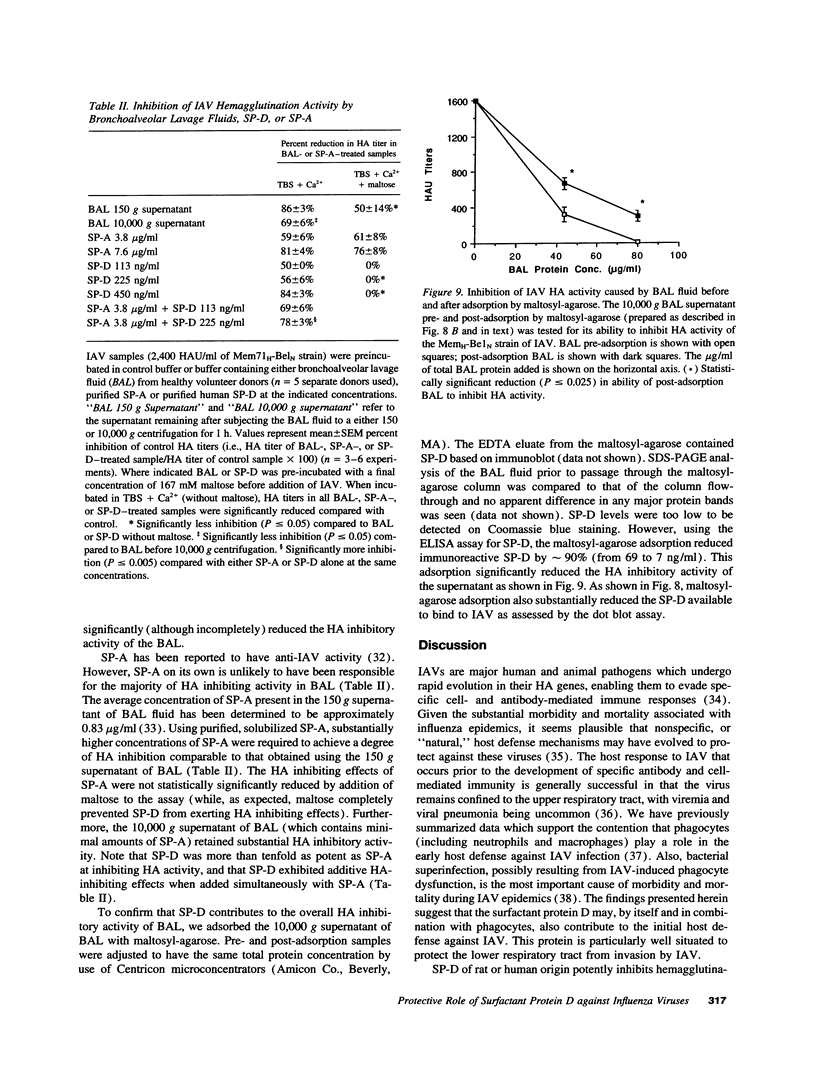
We tested the hypothesis that pulmonary surfactant-associated lectins--surfactant proteins A and D (SP-A, and -D)--contribute to initial protective mechanisms against influenza A viruses (IAVs). SP-D potently inhibited hemagglutination activity of several strains of IAV as well as causing viral aggregation. SP-D enhanced neutrophil binding of IAV and neutrophil respiratory burst responses to the virus. Neutrophil dysfunction resulting from IAV exposure was diminished when the virus was pre-incubated with SP-D. Each of these effects was mediated by the calcium-dependent carbohydrate-binding property of SP-D. Native SP-D preparations of both human and rat origin, as well as recombinant rat SP-D, had similar activity. SP-A also inhibited IAV hemagglutination activity. We have previously reported that related mammalian serum lectins (mannose-binding lectin [MBL] and conglutinin) have similar effects. SP-D was at least 10-fold more potent at causing hemagglutination inhibition than were SP-A or MBL. SP-D was shown to contribute to potent anti-IAV activity of human bronchoalveolar lavage fluid. These results suggest that SP-D--alone, and in conjunction with SP-A and phagocytic cells--constitutes an important component of the natural immune response to IAV infection within the respiratory tract.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Hartley C. A., Jackson D. C. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O., Sørensen A. M., Svehag S. E., Fenouillet E. Conglutinin binds the HIV-1 envelope glycoprotein gp 160 and inhibits its interaction with cell membrane CD4. Scand J Immunol. 1991 Jan;33(1):81–88. doi: 10.1111/j.1365-3083.1991.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Arora D. J., Tremblay P., Bourgault R., Boileau S. Concentration and purification of influenza virus from allantoic fluid. Anal Biochem. 1985 Jan;144(1):189–192. doi: 10.1016/0003-2697(85)90103-4. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Chong C. S., Colbow K. Light scattering and turbidity measurements on lipid vesicles. Biochim Biophys Acta. 1976 Jun 17;436(2):260–282. doi: 10.1016/0005-2736(76)90192-9. [DOI] [PubMed] [Google Scholar]
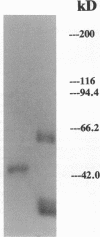
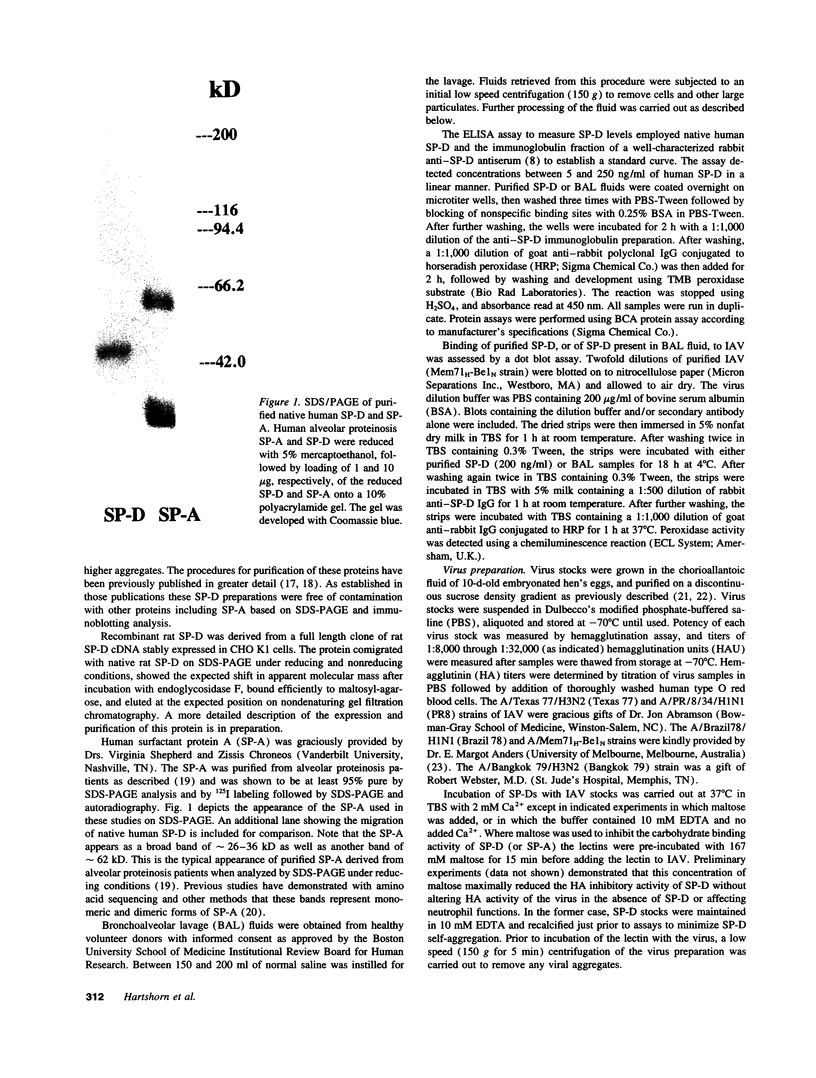
- Crouch E., Persson A., Chang D. Accumulation of surfactant protein D in human pulmonary alveolar proteinosis. Am J Pathol. 1993 Jan;142(1):241–248. [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Persson A., Chang D., Parghi D. Surfactant protein D. Increased accumulation in silica-induced pulmonary lipoproteinosis. Am J Pathol. 1991 Oct;139(4):765–776. [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Kuhlman M., Groopman J. E., Byrn R. A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989 Jan 1;169(1):185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Christiansen P., Thiel S., Svehag S. E., Dessau R., Svendsen P., Andersen O., Laursen S. B., Jensenius J. C. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990 Apr;31(4):453–460. doi: 10.1111/j.1365-3083.1990.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Hartley C. A., Jackson D. C., Anders E. M. Two distinct serum mannose-binding lectins function as beta inhibitors of influenza virus: identification of bovine serum beta inhibitor as conglutinin. J Virol. 1992 Jul;66(7):4358–4363. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K. L., Collamer M., Auerbach M., Myers J. B., Pavlotsky N., Tauber A. I. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988 Aug 15;141(4):1295–1301. [PubMed] [Google Scholar]
- Hartshorn K. L., Collamer M., White M. R., Schwartz J. H., Tauber A. I. Characterization of influenza A virus activation of the human neutrophil. Blood. 1990 Jan 1;75(1):218–226. [PubMed] [Google Scholar]
- Hartshorn K. L., Karnad A. B., Tauber A. I. Influenza A virus and the neutrophil: a model of natural immunity. J Leukoc Biol. 1990 Feb;47(2):176–186. doi: 10.1002/jlb.47.2.176. [DOI] [PubMed] [Google Scholar]
- Hartshorn K. L., Sastry K., Brown D., White M. R., Okarma T. B., Lee Y. M., Tauber A. I. Conglutinin acts as an opsonin for influenza A viruses. J Immunol. 1993 Dec 1;151(11):6265–6273. [PubMed] [Google Scholar]
- Hartshorn K. L., Sastry K., White M. R., Anders E. M., Super M., Ezekowitz R. A., Tauber A. I. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993 Apr;91(4):1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K. L., Tauber A. I. The influenza virus--infected phagocyte. A model of deactivation. Hematol Oncol Clin North Am. 1988 Jun;2(2):301–315. [PubMed] [Google Scholar]
- Kuan S. F., Rust K., Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992 Jul;90(1):97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y., Shiratori M., Ogasawara Y., Tsuzuki A., Akino T. Characterization of pulmonary surfactant protein D: its copurification with lipids. Biochim Biophys Acta. 1991 Nov 5;1086(2):185–190. doi: 10.1016/0005-2760(91)90006-4. [DOI] [PubMed] [Google Scholar]
- Liou L. S., Sastry R., Hartshorn K. L., Lee Y. M., Okarma T. B., Tauber A. I., Sastry K. N. Bovine conglutinin (BC) mRNA expressed in liver: cloning and characterization of the BC cDNA reveals strong homology to surfactant protein-D. Gene. 1994 Apr 20;141(2):277–281. doi: 10.1016/0378-1119(94)90585-1. [DOI] [PubMed] [Google Scholar]
- Lu J., Willis A. C., Reid K. B. Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochem J. 1992 Jun 15;284(Pt 3):795–802. doi: 10.1042/bj2840795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Thiel S., Reid K. B., Sim R. B. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990 Sep 1;172(3):955–959. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack F. X., King T. E., Jr, Voelker D. R., Robinson P. C., Mason R. J. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis. 1991 Jul;144(1):160–166. doi: 10.1164/ajrccm/144.1.160. [DOI] [PubMed] [Google Scholar]
- McNeely T. B., Coonrod J. D. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993 Jan;167(1):91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- Meers P., Mealy T., Pavlotsky N., Tauber A. I. Annexin I-mediated vesicular aggregation: mechanism and role in human neutrophils. Biochemistry. 1992 Jul 21;31(28):6372–6382. doi: 10.1021/bi00143a003. [DOI] [PubMed] [Google Scholar]
- Peterhans E., Mettler F., Manser E. The effect of virus particle size on chemiluminescence induction by influenza and Sendai viruses in mouse spleen cells. Free Radic Res Commun. 1990;11(1-3):11–22. doi: 10.3109/10715769009109663. [DOI] [PubMed] [Google Scholar]
- Ross G. F., Ohning B. L., Tannenbaum D., Whitsett J. A. Structural relationships of the major glycoproteins from human alveolar proteinosis surfactant. Biochim Biophys Acta. 1987 Feb 25;911(3):294–305. doi: 10.1016/0167-4838(87)90070-7. [DOI] [PubMed] [Google Scholar]
- Sastry K., Ezekowitz R. A. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993 Feb;5(1):59–66. doi: 10.1016/0952-7915(93)90082-4. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. IV. Strain-dependent variations. Virology. 1981 Sep;113(2):584–593. doi: 10.1016/0042-6822(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Sweet C., Smith H. Pathogenicity of influenza virus. Microbiol Rev. 1980 Jun;44(2):303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989 Jun 19;250(1):78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Van Iwaarden J. F., Shimizu H., Van Golde P. H., Voelker D. R., Van Golde L. M. Rat surfactant protein D enhances the production of oxygen radicals by rat alveolar macrophages. Biochem J. 1992 Aug 15;286(Pt 1):5–8. doi: 10.1042/bj2860005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Wager R. E., Hawgood S., Dobbs L., Clements J. A. Surfactant apoprotein Mr = 26,000-36,000 enhances uptake of liposomes by type II cells. J Biol Chem. 1987 Feb 25;262(6):2888–2894. [PubMed] [Google Scholar]
- van Iwaarden J. F., van Strijp J. A., Visser H., Haagsman H. P., Verhoef J., van Golde L. M. Binding of surfactant protein A (SP-A) to herpes simplex virus type 1-infected cells is mediated by the carbohydrate moiety of SP-A. J Biol Chem. 1992 Dec 15;267(35):25039–25043. [PubMed] [Google Scholar]