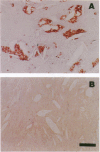
Abstract
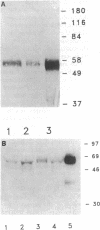
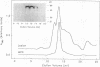
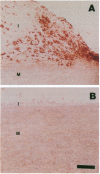
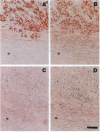
Oxidatively modified lipoproteins have been implicated in atherogenesis, but the mechanisms that promote oxidation in vivo have not been identified. Myeloperoxidase, a heme protein secreted by activated macrophages, generates reactive intermediates that oxidize lipoproteins in vitro. To explore the potential role of myeloperoxidase in the development of atherosclerosis, we determined whether the enzyme was present in surgically excised human vascular tissue. In detergent extracts of atherosclerotic arteries subjected to Western blotting, a rabbit polyclonal antibody monospecific for myeloperoxidase detected a 56-kD protein, the predicted molecular mass of the heavy subunit. Both the immunoreactive protein and authentic myeloperoxidase bound to a lectin-affinity column; after elution with methyl mannoside their apparent molecular masses were indistinguishable by nondenaturing size-exclusion chromatography. Peroxidase activity in detergent extracts of atherosclerotic lesions likewise bound to a lectin column and eluted with methyl mannoside. Moreover, eluted peroxidase generated the cytotoxic oxidant hypochlorous acid (HOCl), indicating that enzymatically active myeloperoxidase was present in lesions. Patterns of immunostaining of arterial tissue with antihuman myeloperoxidase antibodies were similar to those produced by an antimacrophage antibody, and were especially prominent in the shoulder region of transitional lesions. Intense foci of myeloperoxidase immunostaining also appeared adjacent to cholesterol clefts in lipid-rich regions of advanced atherosclerotic lesions. These findings identify myeloperoxidase as a component of human vascular lesions. Because this heme protein can generate reactive species that damage lipids and proteins, myeloperoxidase may contribute to atherogenesis by catalyzing oxidative reactions in the vascular wall.
Full text
PDF

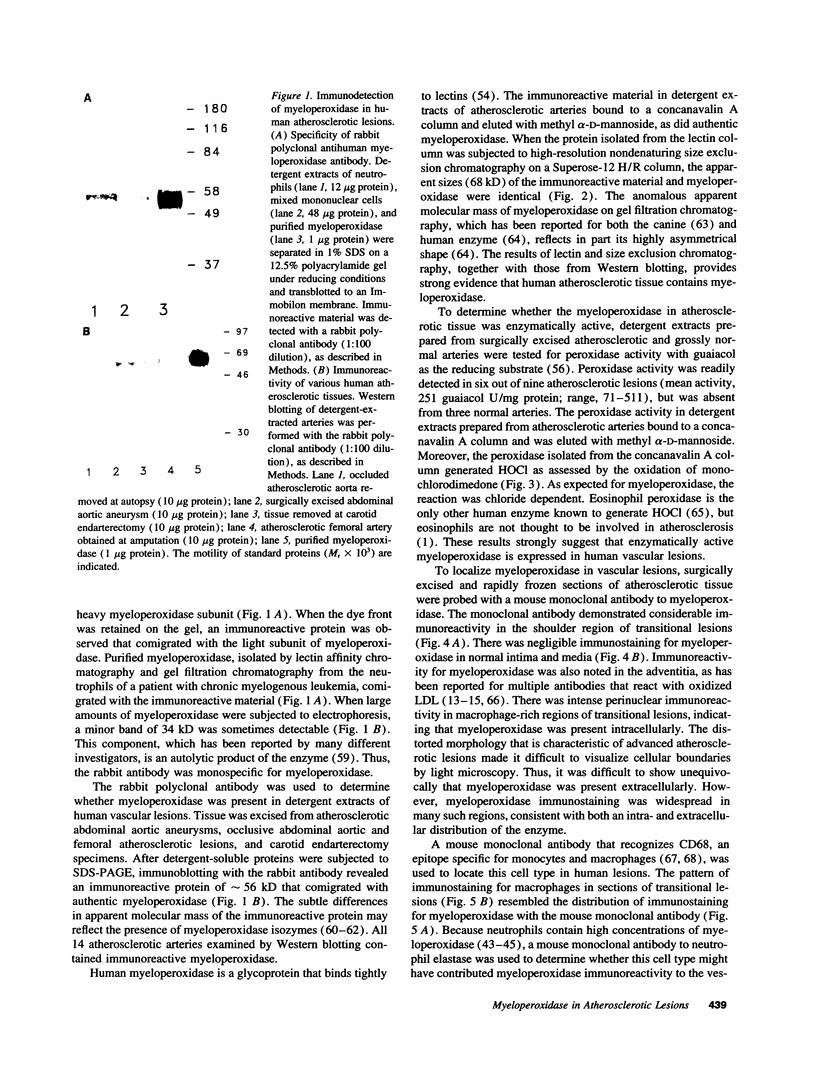
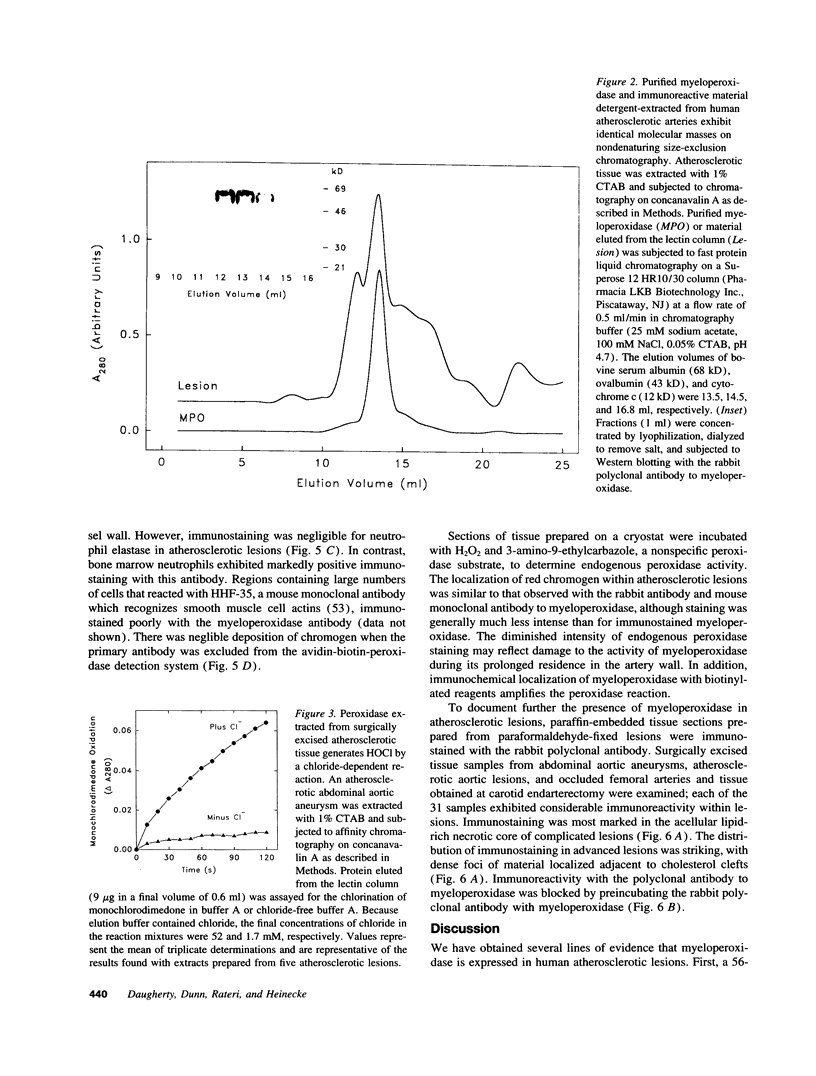
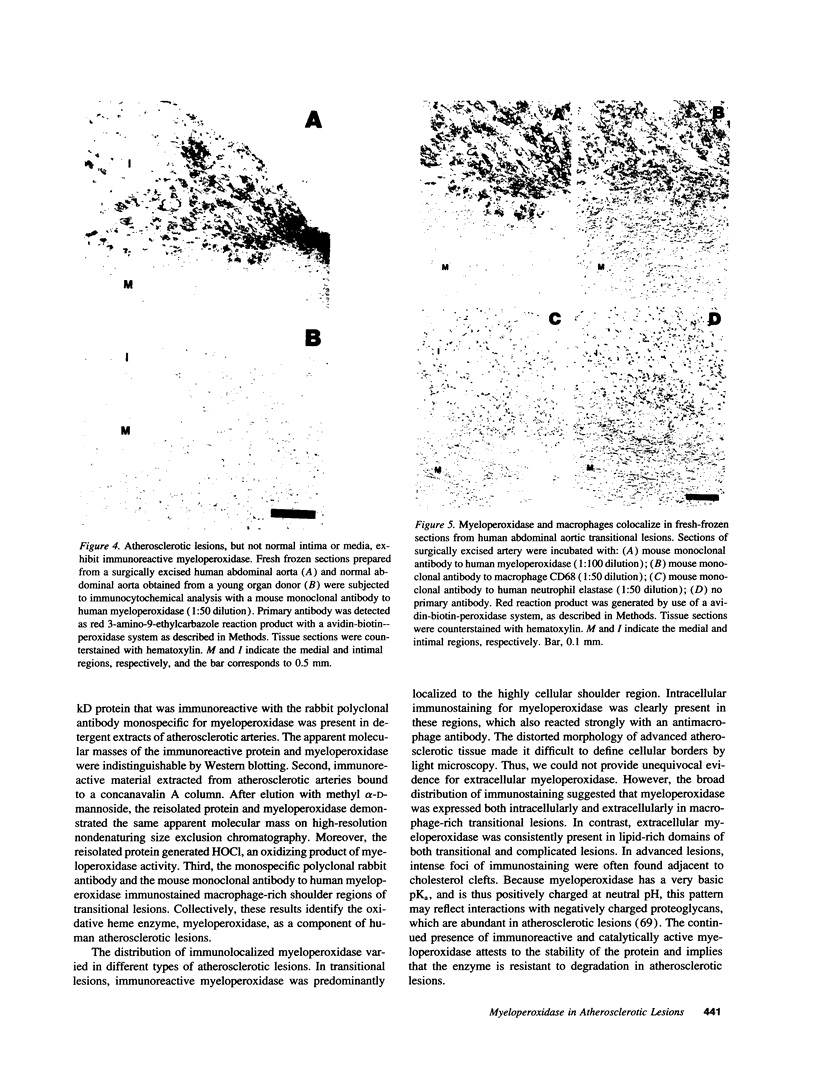



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Arnhold J., Wiegel D., Richter O., Hammerschmidt S., Arnold K., Krumbiegel M. Modification of low density lipoproteins by sodium hypochlorite. Biomed Biochim Acta. 1991;50(8):967–973. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos A., Wever R., Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978 Jul 7;525(1):37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Conrad D. J., Kuhn H., Mulkins M., Highland E., Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br J Pharmacol. 1989 Oct;98(2):612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. Br J Pharmacol. 1991 May;103(1):1013–1018. doi: 10.1111/j.1476-5381.1991.tb12293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Sobel B. E., Schonfeld G. Isolation of low density lipoprotein from atherosclerotic vascular tissue of Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis. 1988 Nov-Dec;8(6):768–777. doi: 10.1161/01.atv.8.6.768. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hannani K., Fei H. H., Lavi S., Berliner J. A. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991 Mar;138(3):601–607. [PMC free article] [PubMed] [Google Scholar]
- Francis G. A., Mendez A. J., Bierman E. L., Heinecke J. W. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6631–6635. doi: 10.1073/pnas.90.14.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J., Wu R., Giscombe R., Holm G., Lefvert A. K., Nilsson J. Induction of T-cell activation by oxidized low density lipoprotein. Arterioscler Thromb. 1992 Apr;12(4):461–467. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Pabalan S., Schultz J. The subunit structure of crystalline canine myeloperoxidase. Biochim Biophys Acta. 1977 Aug 23;493(2):247–259. doi: 10.1016/0005-2795(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hazell L. J., Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J. 1993 Feb 15;290(Pt 1):165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Li W., Daehnke H. L., 3rd, Goldstein J. A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993 Feb 25;268(6):4069–4077. [PubMed] [Google Scholar]
- Heinecke J. W., Li W., Francis G. A., Goldstein J. A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993 Jun;91(6):2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Rosen H., Heinecke J. W., Wolfbauer G., Chait A. Superoxide initiates oxidation of low density lipoprotein by human monocytes. Arteriosclerosis. 1987 Jan-Feb;7(1):55–60. doi: 10.1161/01.atv.7.1.55. [DOI] [PubMed] [Google Scholar]
- Jessup W., Darley-Usmar V., O'Leary V., Bedwell S. 5-Lipoxygenase is not essential in macrophage-mediated oxidation of low-density lipoprotein. Biochem J. 1991 Aug 15;278(Pt 1):163–169. doi: 10.1042/bj2780163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Knapp W., Dörken B., Rieber P., Schmidt R. E., Stein H., von dem Borne A. E. CD antigens 1989. Blood. 1989 Sep;74(4):1448–1450. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Locksley R. M., Nelson C. S., Fankhauser J. E., Klebanoff S. J. Loss of granule myeloperoxidase during in vitro culture of human monocytes correlates with decay in antiprotozoa activity. Am J Trop Med Hyg. 1987 May;36(3):541–548. doi: 10.4269/ajtmh.1987.36.541. [DOI] [PubMed] [Google Scholar]
- Micklem K., Rigney E., Cordell J., Simmons D., Stross P., Turley H., Seed B., Mason D. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989 Sep;73(1):6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Cohen E., Jones P. C., Genco R. J. Evidence for and partial characterization of three major and three minor chromatographic forms of human neutrophil myeloperoxidase. Arch Biochem Biophys. 1986 May 1;246(2):751–764. doi: 10.1016/0003-9861(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Morita Y., Iwamoto H., Aibara S., Kobayashi T., Hasegawa E. Crystallization and properties of myeloperoxidase from normal human leukocytes. J Biochem. 1986 Mar;99(3):761–770. doi: 10.1093/oxfordjournals.jbchem.a135535. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M. Myeloperoxidase deficiency. Hematol Oncol Clin North Am. 1988 Mar;2(1):135–158. [PubMed] [Google Scholar]
- Ogawa T., Koerten H. K., Daems W. T. Peroxidase activity in monocytes and tissue macrophages of mice. Cell Tissue Res. 1978 Apr 28;188(3):361–373. doi: 10.1007/BF00219778. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Wieland E., Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakita R. M., Michel B. R., Rosen H. Myeloperoxidase-mediated inhibition of microbial respiration: damage to Escherichia coli ubiquinol oxidase. Biochemistry. 1989 Apr 4;28(7):3031–3036. doi: 10.1021/bi00433a044. [DOI] [PubMed] [Google Scholar]
- Ramos C. L., Pou S., Britigan B. E., Cohen M. S., Rosen G. M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992 Apr 25;267(12):8307–8312. [PubMed] [Google Scholar]
- Rankin S. M., Parthasarathy S., Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991 Mar;32(3):449–456. [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sepe S. M., Clark R. A. Oxidant membrane injury by the neutrophil myeloperoxidase system. II. Injury by stimulated neutrophils and protection by lipid-soluble antioxidants. J Immunol. 1985 Mar;134(3):1896–1901. [PubMed] [Google Scholar]
- Shepherd V. L., Hoidal J. R. Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am J Respir Cell Mol Biol. 1990 Apr;2(4):335–340. doi: 10.1165/ajrcmb/2.4.335. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Doebber T. W., Olszewski J., Wu M. S., Ventre J., Stevens K. A., Chao Y. S. Low density lipoprotein is protected from oxidation and the progression of atherosclerosis is slowed in cholesterol-fed rabbits by the antioxidant N,N'-diphenyl-phenylenediamine. J Clin Invest. 1992 Jun;89(6):1885–1891. doi: 10.1172/JCI115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Olszewski J. Cellular oxidation of low density lipoprotein is caused by thiol production in media containing transition metal ions. J Lipid Res. 1993 Jul;34(7):1219–1228. [PubMed] [Google Scholar]
- Sparrow C. P., Olszewski J. Cellular oxidative modification of low density lipoprotein does not require lipoxygenases. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):128–131. doi: 10.1073/pnas.89.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck M. J., Khan A. U., Karnovsky M. J. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J Biol Chem. 1992 Jul 5;267(19):13425–13433. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987 Mar 15;262(8):3603–3608. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988 Mar 4;959(1):20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Kukovetz E., Egger G., Schaur R. J. Possible involvement of myeloperoxidase in lipid peroxidation. Int J Biochem. 1992;24(1):121–128. doi: 10.1016/0020-711x(92)90237-u. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Smith A. L. Lipid peroxidation by human blood phagocytes. J Clin Invest. 1974 Sep;54(3):638–645. doi: 10.1172/JCI107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suits A. G., Chait A., Aviram M., Heinecke J. W. Phagocytosis of aggregated lipoprotein by macrophages: low density lipoprotein receptor-dependent foam-cell formation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2713–2717. doi: 10.1073/pnas.86.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. L., Pohl J., Kinkade J. M., Jr Unique autolytic cleavage of human myeloperoxidase. Implications for the involvement of active site MET409. J Biol Chem. 1992 Dec 15;267(35):25282–25288. [PubMed] [Google Scholar]
- Thomas E. L., Jefferson M. M., Grisham M. B. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 1982 Nov 23;21(24):6299–6308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Wever R., Plat H., Hamers M. N. Human eosinophil peroxidase: a novel isolation procedure, spectral properties and chlorinating activity. FEBS Lett. 1981 Jan 26;123(2):327–331. doi: 10.1016/0014-5793(81)80320-1. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., van den Berg J. J., Roitman E., Kuypers F. A. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys. 1992 Aug 1;296(2):547–555. doi: 10.1016/0003-9861(92)90609-z. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]