Abstract
Cholesterol-rich lipid rafts act as signaling microdomains and can regulate receptor function. We have shown in HEK293 cells recombinant P2X1-4 receptors (ATP-gated ion channels) are expressed in lipid rafts. Localization to flotillin-rich lipid rafts was reduced by the detergent Triton X-100. This sensitivity to Triton X-100 was concentration- and subunit-dependent, demonstrating differential association of P2X1-4 receptors with lipid rafts. The importance of raft association to ATP-evoked P2X receptor responses was determined in patch clamp studies. The cholesterol-depleting agents methyl-β-cyclodextrin or filipin disrupt lipid rafts and reduced P2X1 receptor currents by >90%. In contrast, ATP-evoked P2X2-4 receptor currents were unaffected by lipid raft disruption. To determine the molecular basis of cholesterol sensitivity, we generated chimeric receptors replacing portions of the cholesterol-sensitive P2X1 receptor with the corresponding region from the insensitive P2X2 receptor. These chimeras identified the importance of the intracellular amino-terminal region between the conserved protein kinase C site and the first transmembrane segment for the sensitivity to cholesterol depletion. Mutation of any of the variant residues between P2X1 and P2X2 receptors in this region in the P2X1 receptor (residues 20–23 and 27–29) to cysteine removed cholesterol sensitivity. Cholesterol depletion did not change the ATP sensitivity or cell surface expression of P2X1 receptors. This suggests that cholesterol is normally needed to facilitate the opening/gating of ATP-bound P2X1 receptor channels, and mutations in the pre-first transmembrane segment region remove this requirement.
Keywords: ATP, Cholesterol, Lipid Raft, Purinergic Receptor, Receptor Regulation, P2X
Introduction
The concept of ATP acting as an extracellular signaling molecule through P2 receptors was initially proposed by Burnstock in the 1970s (1). It is now clear that ATP is released from a variety of sources, including nerves, astrocytes, and platelets, as well as the result of tissue damage or shear stress. ATP can then activate metabotropic P2Y receptors and ionotropic P2X receptors (2). Seven mammalian P2X receptor subunits (P2X1-7) have been identified. These have two transmembrane domains, intracellular amino and carboxyl termini, a large extracellular ligand binding loop, and form functional trimeric ion channels (3, 4). The membrane topology and subunit stoichiometry of P2X receptors are distinct from both the pentameric cysteine loop and tetrameric glutamate families of ligand-gated ion channels. The homomeric and heteromeric assembly of P2X receptor subunits gives rise to a wide range of phenotypes (3). The use of knock-out animals, antisense strategies, and subtype-selective antagonists has established the contribution of P2X receptors to a range of physiological processes (5–7). For example, P2X1 receptors mediate contraction of smooth muscles; P2X2 receptors are essential for taste sensation; P2X3 receptors contribute to sensory processing, and P2X4 receptors are involved in pain and blood pressure regulation (for reviews see Refs. 4, 5). The regulation of these receptors is therefore of interest as it may have important physiological consequences.
The activity of P2X receptors is sensitive to modulation by accessory proteins (8, 9), and it is likely that P2X receptors are not randomly inserted into the membrane but may be targeted to form signaling complexes. We have previously shown that P2X1 receptors are expressed in lipid rafts (10, 11). These are rich in cholesterol as well as glycosphingolipids and form liquid-ordered microdomains within the liquid-disordered glycerophospholipid bilayer (12, 13). The association of P2X1 receptors with these rafts appears essential for normal function as disruption of the rafts by depletion of membrane cholesterol with methyl-β-cyclodextrin (mβ-CD)3 or sequestration of cholesterol with filipin reduced P2X1 receptor-mediated currents by ∼90%, and responses are restored following mβ-CD treatment with cholesterol repletion (10). However, the molecular basis of this effect remains to be determined. A limited number of studies have looked at the lipid raft association of other P2X receptors with a focus on P2X3 (14–16) and P2X7 receptors (17–19). In this study, we have systematically compared the lipid raft association of recombinant P2X1-4 receptors under a range of conditions and demonstrate that they are differentially distributed. In addition, we compared the effects of lipid raft disruption, and only P2X1 receptors showed a reduction in responsiveness. This enabled us to use chimeras between P2X1 and P2X2 receptors and point mutants to identify the molecular basis of cholesterol sensitivity for the P2X1 receptor.
EXPERIMENTAL PROCEDURES
Membrane Fractionation by Discontinuous Sucrose Density Gradients
P2X1-4 receptors were stably expressed in P2X null HEK293 cells (P2X2-4 cell lines provided by GlaxoSmithKline). Cells were cultured in T175-cm2 tissue flasks and treated with either apyrase (3.2 units/ml, 1 h) (Sigma) or ATP (100 μm, 10 min) (Sigma). Cells were washed with PBS and scraped into 2 ml of 500 mm sodium carbonate, pH 11, and left on ice for 20 min. Alternatively, the cells expressing P2X1-4 subtype receptors were lysed in 2 ml of MBS (25 mm MES and 150 mm NaCl, pH 6.5) containing Triton X-100 (0.1, 0.3, or 1%). All lysates were subjected to three 20-s bursts of sonication and brought to 45% sucrose by addition of an equal volume of 90% sucrose in MBS and loaded in an ultracentrifuge tube. A discontinuous sucrose gradient was layered on top of the sample by placing 4 ml of 35% sucrose prepared in MBS (containing 250 mm Na2CO3) and then 4 ml of 5% sucrose (also in MBS/Na2CO3). The gradient was centrifuged at 39,000 rpm on a TH-641 rotor in a Sorvall OTD65B ultracentrifuge (Kendro Laboratory Products Plc, Bishop's Stortford, UK) for 16 h at 4 °C. After centrifugation, 12 fractions of 1 ml were collected from the top to the bottom of each tube, and Bio-Rad protein assays were performed (Bio-Rad). Samples from fractions 3 to 10 were then equally protein-loaded and run by Western blotting.
Cell Surface Biotinylation
HEK293 cells expressing P2X1 receptors were cultured in 6-well plates and treated with cholesterol-depleting agents (Sigma) as follows: mβ-CD (10 mm, 1 h, room temperature) or filipin (10 μm, 20 min, room temperature). Cells were also treated with the inactive isomer α-cyclodextrin (α-CD) (10 mm, 1 h). Surface proteins were biotinylated by the addition of 0.5 mg/ml sulfo-NHS-LC-biotin (sulfo-biotin; Pierce) diluted in PBS (30 min at 4 °C). Excess biotin was quenched with two 20-min (4 °C) washes with 100 mm glycine in PBS. Cells were lysed in 300 μl of buffer H (100 mm NaCl, 20 mm Tris-Cl, pH 7.4, 1% Triton X-100, and 10 μl/ml protease inhibitor mixture (P8340; Sigma)), incubated on ice for 20 min, and cleared by centrifugation (4 °C at 16,000 × g for 10 min). For isolation of biotinylated proteins, 30 μl of streptavidin-agarose beads (Sigma) was added to 200 μl of supernatant and mixed on a rolling shaker (4 °C, 3 h). The rest of the supernatant was retained to assess total protein for each sample. Beads were washed four times in buffer H, and 30 μl of 2× gel sample loading buffer was added.
Western Blotting
Protein samples were separated on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose. Membranes were then processed with the primary anti-P2X1-4 receptor antibodies (1:1000 for P2X1, -3, and -4, 1:500 for P2X2, Alomone, Jerusalem, Israel), anti-flotillin-2 (1:5000, BD Biosciences), anti-LAMP-1 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), or anti-β-adaptin (1:1000 BD Biosciences). Protein bands were visualized using an ECL Plus kit and Hyperfilm MP (GE Healthcare).
Generation of Chimeric P2X Receptors
Chimeric receptors between the P2X1 and P2X2 receptor were generated using mega-primer-mediated domain swapping. To generate the mega-primers, the smaller of the two receptor components required for the chimera was amplified by PCR. Forward and reverse primers (Sigma) were designed to encode 25 bp of the region of interest and also a 25-bp overhang corresponding to the desired insertion location within the body of the receptor chimera. Each 50-μl PCR contained 50 ng of template P2X receptor, 250 pmol of each primer, 100 μm dNTPs, and 2.5 units/ml PfuTurbo DNA polymerase (Agilent Technologies, Cheshire, UK). PCR parameters included an initial denaturation at 95 °C for 3 min, followed by 30 cycles (95 °C, 30 s; 55 °C, 60 s; and 72 °C, 60 s) and a final elongation at 72 °C for 10 min. The product from this first PCR was purified (Qiagen, Sussex, UK), and 1 μl was used as mega-primer for the second PCR whereby the larger of the two receptor components required for the chimera was used as template DNA (50 ng of template, 1 μl mega-primer, 100 μm dNTPs, and 2.5 units of PfuTurbo DNA polymerase). After initial denaturation at 95 °C for 3 min, DNA fragments were amplified through 16 cycles (95 °C, 30 s; 55 °C, 60 s; and 68 °C, 16 min) followed by elongation at 72 °C for 10 min. An aliquot of the reaction was separated by agarose gel electrophoresis whereby a band corresponding to the size of the chimeric gene could be seen. The reaction was treated with Dpn-1 (Agilent) to remove any remaining template DNA and then transformed into XL1-Blue competent cells (Agilent). The sequence of the resulting clones was verified by sequencing using Protein and Nucleic Acid Chemistry Laboratory services, Leicester University.
Cell Culture and Transfection of HEK293 Cells
Native HEK293 cells were maintained in minimal essential medium with Earle's Salts (with GlutaMAXTM I, Invitrogen) supplemented with 10% fetal bovine serum and 1% nonessential amino acids (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. A monolayer of cells at 80–90% confluence in a 24-well culture dish was transiently transfected using 0.5 μg of DNA (0.45 μg DNA of targeted receptor and 0.05 μg DNA of GFP) and 1 μl of Lipofectamine 2000 (Invitrogen) in 500 μl of serum-free Opti-MEM1. After 24 h of incubation, cells were plated onto 13-mm No. 1 coverslips for electrophysiological experiments and left to grow in DMEM cell culture medium. Cells were subjected to experiments 24–48 h after transfection. Lipofectamine treatment (30 μl added to a T75 flask and incubated for 24 h) had no effect on the raft association of P2X receptors (supplemental Fig. 2).
Electrophysiological Recordings, Solutions, and Data Analysis
Chimeric receptors were initially expressed in Xenopus oocytes, and 100 μm ATP was shown to be a maximal concentration of agonist.4 Whole-cell voltage clamp recordings were made from HEK293 cells using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Membrane currents were recorded at a holding potential of −70 mV (corrected for liquid junction potential). Data were low pass-filtered at 1 kHz, digitized at a sampling interval of 200 μs, and acquired using a Digidata 1200 analog-to-digital converter with pClamp 9.2 acquisition software (Molecular Devices). Microelectrodes were filled with internal solution composed (in mm) of 140 potassium gluconate, 10 EGTA, 10 HEPES, 5 NaCl (pH 7.3, adjusted with KOH) and had resistances in the range of 3–6 megohms. The bath was continuously perfused with extracellular solution containing (in mm) 150 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose (pH 7.3, adjusted with NaOH). ATP (100 μm) was applied with a U-tube perfusion system. In experiments with filipin (10 μm for 20 min), α-CD (10 mm for 1 h), and mβ-CD (10 mm for 1 h), cells were pretreated in drug-containing solution before the experiment. Controls for treated cells were performed on the same day; therefore, the comparisons between nontreated and treated cells used cells from the same batch.
Electrophysiological data were analyzed with CLAMPFIT (Molecular Devices). Data in the text and graphs are shown as mean ± S.E. from n determinations as indicated and analyzed using the unpaired Student's t test, and p < 0.05 was considered significant.
RESULTS
P2X1-4 Receptors Are Associated with Lipid Rafts
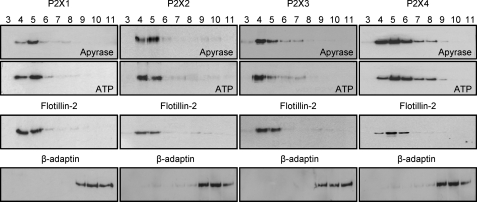
Cholesterol-rich lipid rafts are characterized by the presence of flotillin (20) and can be isolated by ultracentrifugation on a discontinuous sucrose gradient following cell lysis in Na2CO3 MBS (21). The cholesterol-rich rafts are buoyant and are present toward the top of the sucrose gradient. HEK293 cells stably expressing P2X1-4 receptors were used to determine whether these receptors were localized in lipid rafts. To characterize the distribution of P2X receptors under resting conditions, in the absence of agonist, cells were pretreated with apyrase (3.2 units/ml, 1 h) to break down any endogenous ATP. For the HEK293 cells used in this study, flotillin was detected in the upper fractions (Fig. 1) that we have previously shown to be enriched with >75% of the cholesterol (10). The P2X1 receptor was detected in the same fractions as flotillin as we have shown previously (10). A similar distribution and co-localization with flotillin was seen for the P2X2 receptor consistent with its presence in lipid rafts. The P2X3 receptor was predominantly in the lipid raft fractions for 0.1% Triton and in the non-raft fractions when prepared with 0.3 and 1% detergent. In contrast, the P2X4 receptor was co-localized with flotillin in fractions 4–6 consistent with its presence in lipid rafts but was also at high levels in non-raft fractions 7–9 that are characterized by the presence of the non-raft marker β-adaptin (22, 23). This indicates that P2X4 receptors are expressed both within and outside of lipid rafts. The P2X4 receptor can be trafficked through a lysosomal pathway characterized by the presence of the lysosome-associated membrane protein LAMP-1 (24). We were therefore interested to determine whether LAMP-1 was associated with lipid rafts. Western blotting showed that LAMP-1 was only detected in the lipid raft fractions (supplemental Fig. 1).
FIGURE 1.
P2X1-4 receptor association with lipid rafts. HEK293 cells stably expressing P2X1-4 receptors were treated with apyrase (3.2 units/ml, 1 h) or ATP (100 μm, 10 min) and then lysed in Na2CO3 (500 mm, pH 11). Lysates were separated by sucrose density gradients and fractions 3–11 immunoblotted for the appropriate P2X receptor, lipid raft marker flotillin-2, and non-raft marker β-adaptin. Irrespective of the treatment, all subtype P2X receptors were predominantly expressed within the lipid raft fractions (4 and 5) corresponding with the location of flotillin-2. All bands were of the appropriate size (P2X1 ∼55 kDa, P2X2 ∼65 kDa, P2X3 ∼60 kDa, P2X4 ∼50 kDa, flotillin-2 ∼42 kDa, and β-adaptin ∼106 kDa). Blots are representative of those from three separate experiments.
The association of receptors with lipid rafts can be changed following ligand binding. This has been reported for several G protein-coupled receptors, e.g. following activation the β2-adrenoreceptor moves out of the lipid rafts and leads to a reduction in signaling (25, 26). Activation of P2X1 and P2X3 receptors results in desensitization, and ∼5 min are required following agonist removal for responses to recover. We therefore tested whether movement out of the lipid rafts could contribute to desensitization. For P2X1-4 receptors, stimulation with ATP (100 μm) had no effect on the receptor distribution (Fig. 1). This demonstrates that in contrast to some G-protein-coupled receptors, the activation of P2X1-4 ligand-gated ion channel receptors does not result in movement out of the lipid rafts.
Differential Lipid Raft Association of P2X Receptors Revealed by Triton X-100 Sensitivity
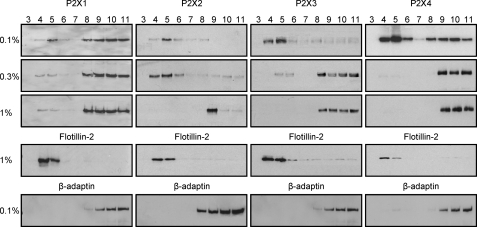
Lipid rafts show heterogeneity based on their protein and lipid content (12), and differences in detergent solubility can be used to characterize different raft domains as well as the strength of association of a particular protein with the rafts. The use of Na2CO3 MBS (see Fig. 1) gives rise to lipid rafts that are enriched in glycerophospholipids and retain inner leaflet membrane lipids (12). In contrast, lysis using the detergent Triton X-100 leads to a depletion of glycerophospholipids and disruption of inner leaflet membrane lipids (12). We have therefore compared the raft association of P2X1-4 receptors following solubilization in 0.1, 0.3, and 1% Triton X-100. Flotillin was detected in the buoyant fractions 4–5 for Triton X-100 at 1% showing that the core raft is maintained under these conditions. Triton X-100 solubilization resulted in the predominant component of the P2X1 receptor being detected in the non-raft fractions. However, some P2X1 receptors were still detected in the raft fraction at 1% Triton consistent with our previous study (Fig. 2) (10). The P2X2 receptor was associated with flotillin for extraction in 0.1 and 0.3% Triton, but when this was increased to 1%, P2X2 moved to the non-raft fractions and was below the limit of detection in the lipid raft fractions (Fig. 2). The P2X3 receptor was predominantly in the lipid raft fractions for 0.1% Triton and was only detected in the non-raft fractions when prepared with 0.3 and 1% detergent (Fig. 2). This contrasts with the localization of P2X3 receptors in lipid rafts following 1% Triton X treatment in cerebellar granule neurons (14) and suggests that differences in lipid composition or the repertoire of accessory proteins differs between HEK293 cells and granule cells. The P2X4 receptor showed both raft and non-raft association with 0.1% Triton and no raft association at concentrations of Triton at ≥0.3% (Fig. 2). These results demonstrate that P2X1-4 receptors show differential distribution with lipid rafts.
FIGURE 2.
Differential sensitivity of P2X1-4 receptor association with lipid rafts revealed by Triton X-100 detergent. HEK293 cells stably expressing P2X1-4 receptors were lysed in the presence of 0.1, 0.3, or 1% Triton X-100. Lysates were separated by sucrose density gradients, and fractions 3–11 were immunoblotted for the appropriate P2X receptor, lipid raft marker flotillin-2, and non-raft marker β-adaptin. All concentrations of Triton X-100 partially redistributed the P2X1 receptor along the sucrose gradient with only a small percentage remaining within the lipid raft fractions (4 and 5 as indicated by flotillin-2). In contrast, subtype receptors P2X2-4 completely redistributed to outside of the lipid rafts (as indicated by β-adaptin) with increasing concentrations of Triton X-100. All bands were of the appropriate size (P2X1 ∼55 kDa, P2X2 ∼65 kDa, P2X3 ∼60 kDa, P2X4 ∼50 kDa, flotillin-2 ∼42 kDa, and β-adaptin ∼106 kDa). Blots are representative of those from three separate experiments.
Effect of Lipid Raft Disruption on P2X Receptor Properties
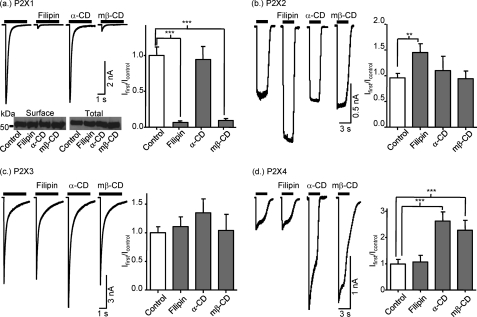
The co-localization of P2X1-4 receptors with flotillin clearly shows that these receptors are associated with lipid rafts; however, the extent of the association appears subtype-dependent. To determine whether the lipid rafts play a regulatory role on the P2X1-4 receptor function, we tested the effects of disrupting the lipid rafts either by using filipin (10 μm for 20 min) to form multimeric globular complexes with membrane cholesterol or depleting cholesterol with mβ-CD (10 mm for 1 h). ATP (100 μm) evoked transient inward P2X1 receptor currents as described previously (10). Filipin or mβ-CD treatment reduced the peak amplitude of P2X1 receptor currents by >90% (Fig. 3a). The inactive α-CD (10 mm for 1 h) had no effect on P2X1 receptor currents indicating that the effects of mβ-CD result from cholesterol depletion (Fig. 3a). The reduction in P2X1 receptor responses could result from a decrease in agonist sensitivity, surface expression, and/or an effect on channel gating. The time course of ATP-evoked P2X1 receptor currents is concentration-dependent, and the lack of effect of filipin or mβ-CD treatment on the rise time or desensitization of the P2X1 receptor currents (data not shown) indicates that the reduction in current amplitude does not result from a reduction in agonist sensitivity. Cell surface expression of the P2X1 receptor was also unaffected by cholesterol disruption (Fig. 3a, lower panel) demonstrating that a change in receptor number does not account for the inhibition of P2X1 receptor responses. Taken together, these results suggest that cholesterol depletion has an effect on P2X1 receptor channel gating/activation. These results are consistent with our previous work demonstrating the importance of lipid rafts in the regulation of P2X1 receptors (10, 11).
FIGURE 3.
Selective inhibition of P2X1 receptors by lipid raft disruption. Effects of lipid raft disruption by filipin (10 μm for 20 min) and mβ-CD (10 mm for 1h) were tested for currents mediated by P2X1, P2X2, P2X3, and P2X4 receptors expressed in HEK293 cells (panels a–d), respectively). α-CD (10 mm for 1 h) was used as an inactive analog of mβ-CD. Currents were evoked by fast application of ATP (100 μm) for 3 s with a U-tube application system. We compared the densities of the first currents in treated and nontreated cells (histograms on the right side of panels). Surface biotinylation revealed that treatment with filipin, α-CD, or mβ-CD does not change the surface expression of P2X1 receptors (panel a). **, p < 0.01; ***, p < 0.001.
At P2X2 receptors ATP (an EC50 concentration of 10 μm or a maximal concentration of 100 μm) evoked inward currents that were sustained during the 3 s of application and unaffected by mβ-CD treatment (Fig. 3b and supplemental Fig. 3) demonstrating that the integrity of lipid rafts is not essential for normal P2X receptor-mediated responses. Filipin treatment resulted in an ∼50% increase in current amplitude for the P2X2 receptor (Fig. 3b). The lack of effect of mβ-CD on P2X2 receptors suggests that cholesterol depletion does not affect the P2X2 receptor. Whether the potentiation by filipin could result from its clustering/chelation of cholesterol in the membrane or an effect not related to changes in cholesterol remains to be determined. Rapid transient inward currents were evoked by ATP (100 μm, a maximal concentration) from HEK293 cells expressing P2X3 receptors. The amplitude and time course of these responses were unaffected by filipin or cyclodextrin treatment (Fig. 3c). Similarly, responses to an ∼EC50 concentration of ATP (1 mm) were unaffected by mβ-CD treatment (supplemental Fig. 3). These results are consistent with previous studies on native and recombinant P2X3 receptors (15, 16). Filipin treatment had no effect on ATP-evoked P2X4 receptor currents evoked either by an EC50 concentration of ATP (10 μm) or a maximal concentration (100 μm) (Fig. 3 and supplemental Fig. 3), indicating that lipid rafts do not play a role in their basal regulation. However, P2X4 receptor currents were potentiated ∼2.5-fold following treatment with cyclodextrins. The similar enhancement of the response by either mβ-CD, which depletes cholesterol, or the α-CD analog, which has no effect on cholesterol (as well as the lack of effect of filipin), suggests that the potentiation is due to an effect of the cyclodextrin family of compounds and not due to an effect on cholesterol. The level of P2X4 receptor expression and functional currents can be regulated by lysosome trafficking and is potentiated by ionomycin (24) and methylamine (27). This raises the possibility that cyclodextrins potentiate P2X4 currents by an effect on receptor trafficking.
Molecular Basis of P2X1 Receptor Sensitivity to Cholesterol Depletion
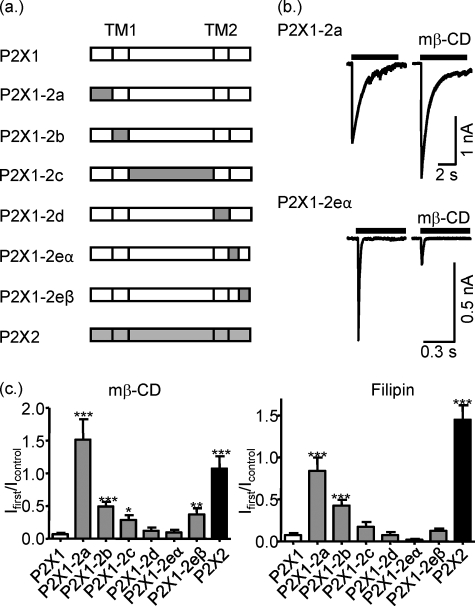
This study demonstrates that although P2X1-4 receptors are present in lipid rafts, albeit to different extents, only P2X1 receptor currents are sensitive to cholesterol depletion. To address the molecular basis of cholesterol sensitivity, we generated a series of chimeras on a P2X1 receptor background replacing the amino terminus (P2X1-2a), the first transmembrane segment (TM1) (P2X1-2b), the extracellular domain (P2X1-2c), the second transmembrane segment (TM2) (P2X1-2d), or the regions of the carboxyl terminus (P2X1-2eα, residues 354–366 and P2X1-2eβ residues 366 onwards swapped) with the equivalent region from the human P2X2 receptor (Fig. 4a).
FIGURE 4.
Sensitivity of P2X1-2 chimeras to cholesterol depletion. a, schematic representation of wild type of P2X1 and P2X2 receptors and their chimeric constructs. b, representative currents mediated by P2X1-2a and P2X1-2eα chimeric receptors in HEK293 cells in control conditions and after treatment with mβ-CD. Currents were induced by application of ATP (100 μm) for 5 s. c, summary of the effects of mβ-CD or filipin treatment on currents mediated by P2X1, P2X2, and P2X1/P2X2 chimeric receptors as fraction of the response to ATP in nontreated cells (n = 5–17). t test shows that effects of mβ-CD and filipin treatment on currents mediated by P2X1 and all P2X1/P2X2 chimeric receptors (but P2X1-2a in case of mβ-CD) were significantly different from P2X2 (not shown on figure). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To determine which regions of the P2X1 receptor contribute to cholesterol sensitivity, the chimeric receptors were transiently expressed in HEK293 cells, and the effects of cholesterol depletion with mβ-CD or filipin were determined on the responses to a maximal concentration of ATP (100 μm). Replacement of TM2 (P2X1-2d) or the first half of the carboxyl terminus of the P2X1 receptor (P2X1-2eα) with the P2X2 receptor had no effect on the reduction of the responses following cholesterol depletion (Fig. 4, b and c). Swapping the P2X2 receptor TM1 (P2X1-2b), extracellular domain (P2X1-2c), or second half of the carboxyl terminus (P2X1-2eβ) for the equivalent P2X1 receptor region reduced the effects of cholesterol depletion by ∼20–40% suggesting that these regions contribute to receptor regulation by lipid rafts. However, replacing the amino terminus of the P2X1 receptor with the equivalent region from the P2X2 receptor (P2X1-2a) abolished the inhibitory effect of cholesterol depletion (Fig. 4, b and c). The “mirror” chimera inserting the amino terminus of the P2X1 receptor into the P2X2 receptor (P2X2-1a) was reduced following filipin treatment (145 ± 17 and 23 ± 3% of untreated cells for P2X2 and P2X2-1a, respectively, n = 7, p < 0.001). These results demonstrate that the cholesterol sensitivity of the P2X1 receptor is predominantly determined by the intracellular amino terminus.
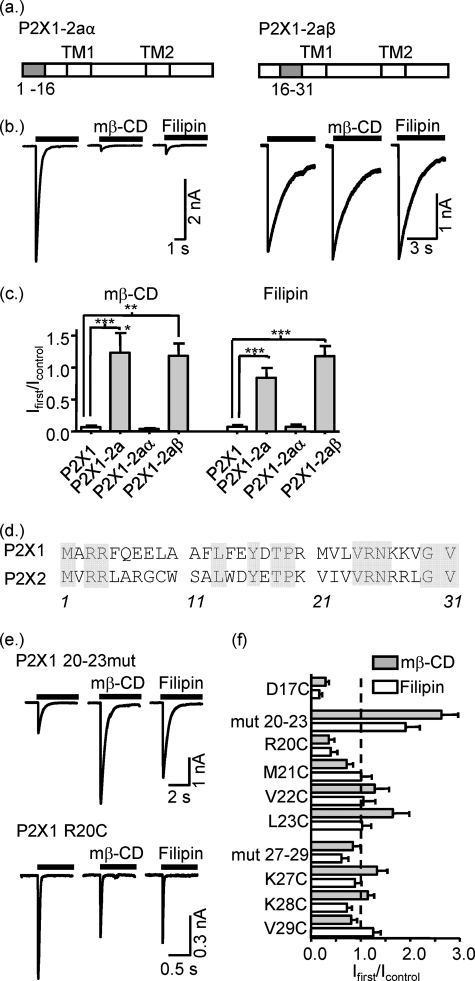
To characterize further the contribution of the amino terminus of the P2X1 receptor to cholesterol sensitivity, two additional chimeras were generated, P2X1-2aα and P2X1-2aβ, that subdivided the amino-terminal replacement (Fig. 5a). When the first 16 amino acids of the P2X1 receptor amino terminus were replaced with those from the P2X2 receptor, the currents recorded from the resulting chimera (P2X1-2aα) were abolished following cholesterol depletion by either mβ-CD or filipin treatment (Fig. 5a). In contrast, cholesterol depletion had no effect on ATP-evoked currents recorded from the P2X1-2aβ chimera (second half of the amino-terminal residues 16–31 of the P2X2 receptor) (Fig. 5a). Western blot analysis showed that this chimera showed the same raft association as the WT P2X1 receptor (supplemental Fig. 2). Analysis of the amino acid sequence for P2X1 and P2X2 receptors in this region showed that there are eight differences, residue 17 and two clusters of residues 20–23 and 27–29 (P2X1 receptor numbering). Replacement of either of the clusters in P2X1 with the P2X2 receptor sequence produced channels that were either insensitive to cholesterol depletion P2X1-mut27–29 or actually potentiated P2X1-mut20–23 (Fig. 5f). We have previously used cysteine-scanning mutagenesis of the intracellular region Tyr16–Gly30 to investigate the basis of P2X1 receptor regulation by G-protein-coupled receptors and phorbol ester (28). In this study, we used the individual cysteine mutants of the eight variant amino acids to determine their contribution to cholesterol sensitivity. Six of the eight mutants (M21C, V22C, L23C, K27C, K28C, and V29C) were insensitive to cholesterol depletion, and for D17C and R20C cholesterol depletion resulted in only an ∼30–40% reduction in peak current amplitude, respectively (Fig. 5). As described previously (28, 29), some of the chimeras and point mutants had an effect on the time course of receptor desensitization, e.g. an ∼2-fold slowing in desensitization on swapping of the intracellular amino terminus (P2X1-2a). However, there was no correlation between the time course of response and cholesterol sensitivity (supplemental Fig. 1). These results highlight the importance of multiple amino acids in the region of the amino terminus just before TM1 in cholesterol regulation of the P2X1 receptor.
FIGURE 5.
Identification of the regions in the amino terminus of the P2X1 receptor contributed to lipid raft disruption sensitivity. a, schematic representation of P2X1-2aα and P2X1-2aβ chimera constructs where 1–16 and 16–31 residues from the P2X1 receptor were swapped for P2X2 ones, respectively. b, representative currents mediated by P2X1-2aα and P2X1-2aβ chimeric receptors in HEK293 cells in control conditions and after treatment with mβ-CD or filipin. Currents were induced by application of ATP (100 μm) for 5 s. c, summary of the effects of mβ-Cd and filipin treatment on currents mediated by P2X1, P2X1-2a, P2X1-2aα, and P2X1-2aβ chimeric receptors as fraction of the response to ATP in nontreated cells (n = 6–14). d, amino acid sequences of P2X1 and P2X2 intracellular amino termini. Conserved amino acids are highlighted gray. e, representative currents mediated by P2X1(20–23) and P2X1 R20C mutants evoked by application of ATP in control conditions and after treatment with mβ-CD or filipin. f, summary of the effects of mβ-CD and filipin treatment on currents mediated by P2X1 mutants (n = 5–10). All of them were significantly different from the P2X1 receptor wild type (not shown on figure). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
Cholesterol- and sphingolipid-rich lipid rafts form microdomains within the cell membrane that can have important signaling roles. The lipid rafts are buoyant and can be isolated by density fractionation on sucrose gradients. P2X1-4 receptors were detected in the lipid raft fractions characterized by their buoyant density and the presence of the lipid raft marker protein flotillin. Different classes of lipid rafts have been identified based on their lipid/protein composition, and raft association can be characterized further by determining the sensitivity to detergent extraction (for a review see Ref. 12). Raft association of P2X1-4 receptors decreased as the concentration of the detergent Triton X was increased and the sensitivity was dependent on the P2X receptor subtype. For example using 0.3% Triton X-100, P2X2 receptors but not P2X3 receptors were located in the lipid rafts. These results demonstrate that P2X receptors show differential subtype-dependent associations with lipid rafts. They also demonstrate the importance of comparing a range of conditions as they isolate rafts with different properties (12). For example 1% Triton X-100 extraction indicates that P2X2 and P2X4 receptors are not expressed in lipid rafts as described previously (14); however, lower concentrations of Triton or Na2CO3 MBS-based methods clearly demonstrate raft association of these receptors. The Triton X sensitivity of P2X receptor association with lipid rafts is similar to that reported for other proteins (12), for example the T cell receptor (30). What might explain this sensitivity to the extraction method? The Na2CO3 MBS extraction method maintains high levels of glycerophospholipids and retention of inner leaflet membrane lipids that are depleted when Triton X detergent extraction is used (for review see Ref. 12). This suggests that for P2X1-4 receptors glycerophospholipids and/or inner leaflet membrane lipids play an essential role in raft association.
Cholesterol is found in the plasma membrane and plays an essential role in the lipid rafts and the maintenance of these signaling micro-domains. This study shows that although P2X1-4 receptors were found in lipid rafts, this association only plays an important role in regulation of P2X1 receptors under basal conditions. P2X2-4 receptors have been shown to be regulated by second messenger pathways (31–33). Raft association can help organize signaling complexes, and it remains to be determined if localization to rafts is essential for the regulation of P2X2-4 receptors.
The expression of P2X1-4 receptors in lipid rafts suggests that they may share a common core method of raft association. Common raft association signals appear absent from P2X1-4 receptors. For example, palmitoylation or myristoylation can target proteins to lipid rafts (34, 35), but such post-translational modifications have not been reported for P2X1-4 receptors. Similarly, association with caveolin promotes lipid raft association; however, caveolin-binding motifs (36) are absent from the P2X1-4 receptors. This raises the possibility that P2X receptors have a novel raft association motif or that raft association is mediated by P2X receptor-interacting proteins. For example, the heat shock protein Hsp90 is found in lipid rafts (37) and can be co-purified with P2X2 and P2X7 receptors (9, 38).
The differential association of P2X1-4 receptors with lipid rafts, highlighted by their Triton X sensitivity, suggests that nonconserved residues/regions are also likely to be involved in lipid raft association. Direct binding of cholesterol to TM domains has been shown for voltage-dependent anion channels (39) and suggested for the nicotinic acetylcholine receptor (40) and is required for the functional expression of both channels (39, 40). At β2-adrenoceptors, a highly conserved aromatic tryptophan residue in the transmembrane helix contributes significantly to interaction with cholesterol (41). Interestingly, there are two conserved aromatic residues in TM1 of P2X receptors (Tyr43 and Trp47, P2X1 receptor numbering) raising the possibility that they could be involved in cholesterol binding in the membrane and raft association. Alanine mutants of the conserved tyrosine residue have been studied at P2X1-4 receptors. At the P2X1 receptor, the Y43A mutant had no effect on receptor expression but rendered the receptor nonfunctional (42). The equivalent mutations in P2X2-4 receptors had no effect on responses to a maximal concentration of ATP (42). This pattern is the same as that described in this study for the effects of cholesterol disruption (P2X1 receptors sensitive and no effects on P2X2-4 receptors), and it raises the possibility that a reduction in cholesterol binding/raft association, because of the removal of the conserved aromatic residue, underlies the effects of the mutation at the P2X1 receptor. The chimera in which the TM1 segment was swapped between P2X2 and P2X1 retains the conserved residues and yet still showed sensitivity to cholesterol depletion (albeit reduced when TM1 of P2X1 was replaced with that of P2X2 receptor; chimera P2X1-2b). This suggests that additional parts of the receptor play a substantial role in lipid raft regulation of the P2X1 receptor.
The detergent sensitivity of raft association suggests that inner leaflet lipids may play an important role (see above) in the raft sensitivity of P2X1 receptors by binding to the intracellular amino- and/or carboxyl-terminal domains of the receptor. The membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) interacts with the intracellular carboxyl terminus of Kir2.1 potassium channels and plays a central role in regulation of the channel by cholesterol (43). PIP2 binds to the P2X1 receptor at the intracellular carboxyl terminus close to TM2, and P2X1 receptor currents were inhibited following PIP2 depletion with wortmannin (44), raising the possibility that this region may contribute to cholesterol sensitivity. However, PIP2 also binds to the carboxyl terminus of P2X2 receptors (45), and depletion of PIP2 by wortmannin inhibits not only P2X1 receptor-mediated currents (43) but also those from cholesterol-insensitive P2X2 (45) and P2X4 (33) receptors. In addition, the chimera P2X1-2eα that swapped the PIP2-sensitive region had no effect on cholesterol sensitivity. These results suggest that the carboxyl-terminal region adjacent to TM2 does not regulate P2X1 receptor cholesterol sensitivity.
The lack of effect of cholesterol depletion on the chimera P2X1-2aβ demonstrated an essential role of amino acids 16–30 in cholesterol regulation of the P2X1 receptor. This run of amino acids connects the conserved protein kinase C site found in all P2X receptors (46) with the TM1. However structural information on this region is not available as the amino and carboxyl termini of the zebrafish P2X4 receptor were truncated to optimize its crystallization (47). Further mini-chimeras of P2X1-mut20–23 and P2X1-mut27–29 also removed the inhibitory effects of cholesterol depletion as did individual cysteine point mutations of the variant amino acids. It is interesting that of the substitutions between P2X1 and P2X2, receptors in these regions are rather conservative and maintain the positive charge or nonpolar nature of the side chain. These results indicate it is the integrity of both these regions that is important for the regulatory effects of cholesterol, and minor conservative changes disrupt the interaction between the regions 20–23 and 27–29 (P2X1 receptor numbering). The interaction of these regions is similar to what we have reported previously where the potentiation of P2X1 receptor currents by phorbol ester was reduced for the mutants R20C, M21C, K27C, and V29C (28) and further supports an important role of interaction of these regions in channel regulation.
How might the pre-TM1 region regulate cholesterol sensitivity? The reduction in P2X1 receptor currents following cholesterol depletion could result from a decrease in channel expression, reduced agonist sensitivity, and/or an effect on channel opening/gating. The first two of these mechanisms seem unlikely as (i) the lack of effect of mβ-CD or filipin treatment on the surface expression of P2X1 receptors demonstrates channel expression is unaffected, and (ii) the time course of P2X1 receptor currents is concentration-dependent, and the reduction in current amplitude by cholesterol depletion with no effect on time course rules out a reduction in agonist sensitivity. This suggests that the pre-TM1 region of the receptor plays a role in the regulation of the opening of agonist-bound P2X1 receptors, i.e. an effect on channel gating. A similar requirement of cholesterol for the function of voltage-dependent anion channel and nicotinic acetylcholine receptors has been reported (39, 40). The mutations in the P2X1 receptor pre-TM1 region could act to stabilize the receptor and remove the cholesterol sensitivity of the gating. Whether cholesterol acts directly to stabilize the conformation of the P2X1 receptor or association with lipid rafts favors interaction with inner leaflet lipids that regulate the channel remains to be determined.
Supplementary Material
Acknowledgments
We thank M. P. Mahaut Smith for comments on the manuscript and C. Dart for suggesting the use of β-adaptin.
This work was supported by the Wellcome Trust.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
R. C. Allsopp, U. Lalo, and R. J. Evans, unpublished observations.
- mβ-CD
- methyl-β cyclodextrin
- TM1
- first transmembrane segment
- α-CD
- α-cyclodextrin
- TM2
- second transmembrane segment
- PIP2
- phosphatidylinositol 4,5-bisphosphate.
REFERENCES
- 1.Burnstock G. (2006) Trends Pharmacol. Sci. 27, 166–176 [DOI] [PubMed] [Google Scholar]
- 2.Ralevic V., Burnstock G. (1998) Pharmacol. Rev. 50, 413–492 [PubMed] [Google Scholar]
- 3.North R. A. (2002) Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 4.Roberts J. A., Vial C., Digby H. R., Agboh K. C., Wen H., Atterbury-Thomas A., Evans R. J. (2006) Pflugers Arch. 452, 486–500 [DOI] [PubMed] [Google Scholar]
- 5.Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 [DOI] [PubMed] [Google Scholar]
- 6.Khakh B. S., North R. A. (2006) Nature 442, 527–532 [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. (2006) Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 8.Kim M., Jiang L. H., Wilson H. L., North R. A., Surprenant A. (2001) EMBO J. 20, 6347–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaumont S., Compan V., Toulme E., Richler E., Housley G. D., Rassendren F., Khakh B. S. (2008) Sci. Signal. 1, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vial C., Evans R. J. (2005) J. Biol. Chem. 280, 30705–30711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vial C., Fung C. Y., Goodall A. H., Mahaut-Smith M. P., Evans R. J. (2006) Biochem. Biophys. Res. Commun. 343, 415–419 [DOI] [PubMed] [Google Scholar]
- 12.Pike L. J. (2004) Biochem. J. 378, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia F., Gao X., Kwan E., Lam P. P., Chan L., Sy K., Sheu L., Wheeler M. B., Gaisano H. Y., Tsushima R. G. (2004) J. Biol. Chem. 279, 24685–24691 [DOI] [PubMed] [Google Scholar]
- 14.Vacca F., Amadio S., Sancesario G., Bernardi G., Volonté C. (2004) J. Neurosci. Res. 76, 653–661 [DOI] [PubMed] [Google Scholar]
- 15.Liu M., Huang W., Wu D., Priestley J. V. (2006) Eur. J. Neurosci. 24, 1–6 [DOI] [PubMed] [Google Scholar]
- 16.Vacca F., Giustizieri M., Ciotti M. T., Mercuri N. B., Volonté C. (2009) J. Neurochem. 109, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Marcos M., Pérez-Andrés E., Tandel S., Fontanils U., Kumps A., Kabré E., Gómez-Muñoz A., Marino A., Dehaye J. P., Pochet S. (2006) J. Lipid Res. 47, 705–714 [DOI] [PubMed] [Google Scholar]
- 18.Barth K., Weinhold K., Guenther A., Young M. T., Schnittler H., Kasper M. (2007) FEBS J. 274, 3021–3033 [DOI] [PubMed] [Google Scholar]
- 19.Gonnord P., Delarasse C., Auger R., Benihoud K., Prigent M., Cuif M. H., Lamaze C., Kanellopoulos J. M. (2009) FASEB J. 23, 795–805 [DOI] [PubMed] [Google Scholar]
- 20.Bickel P. E., Scherer P. E., Schnitzer J. E., Oh P., Lisanti M. P., Lodish H. F. (1997) J. Biol. Chem. 272, 13793–13802 [DOI] [PubMed] [Google Scholar]
- 21.Ostrom R. S. (2002) Mol. Pharmacol. 61, 473–476 [DOI] [PubMed] [Google Scholar]
- 22.Jacobs C., Onnockx S., Vandenbroere I., Pirson I. (2004) FEBS Lett. 565, 70–74 [DOI] [PubMed] [Google Scholar]
- 23.Pagano M., Clynes M. A., Masada N., Ciruela A., Ayling L. J., Wachten S., Cooper D. M. (2009) Am. J. Physiol. Cell Physiol. 296, C607–C619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi O. S., Paramasivam A., Yu J. C., Murrell-Lagnado R. D. (2007) J. Cell Sci. 120, 3838–3849 [DOI] [PubMed] [Google Scholar]
- 25.Rybin V. O., Xu X., Lisanti M. P., Steinberg S. F. (2000) J. Biol. Chem. 275, 41447–41457 [DOI] [PubMed] [Google Scholar]
- 26.Ostrom R. S., Gregorian C., Drenan R. M., Xiang Y., Regan J. W., Insel P. A. (2001) J. Biol. Chem. 276, 42063–42069 [DOI] [PubMed] [Google Scholar]
- 27.Toulme E., Garcia A., Samways D., Egan T. M., Carson M. J., Khakh B. S. (2010) J. Gen Physiol. 135, 333–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen H., Evans R. J. (2009) J. Neurochem. 108, 331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner P., Seward E. P., Buell G. N., North R. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15485–15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janes P. W., Ley S. C., Magee A. I. (1999) J. Cell Biol. 147, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boué-Grabot E., Archambault V., Séguéla P. (2000) J. Biol. Chem. 275, 10190–10195 [DOI] [PubMed] [Google Scholar]
- 32.Paukert M., Osteroth R., Geisler H. S., Brandle U., Glowatzki E., Ruppersberg J. P., Gründer S. (2001) J. Biol. Chem. 276, 21077–21082 [DOI] [PubMed] [Google Scholar]
- 33.Bernier L. P., Ase A. R., Chevallier S., Blais D., Zhao Q., Boué-Grabot E., Logothetis D., Séguéla P. (2008) J. Neurosci. 28, 12938–12945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fragoso R., Ren D., Zhang X., Su M. W., Burakoff S. J., Jin Y. J. (2003) J. Immunol. 170, 913–921 [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A., Arnaud L., Cooper J. A. (2003) J. Biol. Chem. 278, 40806–40814 [DOI] [PubMed] [Google Scholar]
- 36.Couet J., Li S., Okamoto T., Ikezu T., Lisanti M. P. (1997) J. Biol. Chem. 272, 6525–6533 [DOI] [PubMed] [Google Scholar]
- 37.Waheed A. A., Jones T. L. (2002) J. Biol. Chem. 277, 32409–32412 [DOI] [PubMed] [Google Scholar]
- 38.Adinolfi E., Kim M., Young M. T., Di Virgilio F., Surprenant A. (2003) J. Biol. Chem. 278, 37344–37351 [DOI] [PubMed] [Google Scholar]
- 39.Hiller S., Garces R. G., Malia T. J., Orekhov V. Y., Colombini M., Wagner G. (2008) Science 321, 1206–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalziel A. W., Rollins E. S., McNamee M. G. (1980) FEBS Lett. 122, 193–196 [DOI] [PubMed] [Google Scholar]
- 41.Hanson M. A., Cherezov V., Griffith M. T., Roth C. B., Jaakola V. P., Chien E. Y., Velasquez J., Kuhn P., Stevens R. C. (2008) Structure 16, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jindrichova M., Vavra V., Obsil T., Stojilkovic S. S., Zemkova H. (2009) J. Neurochem. 109, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epshtein Y., Chopra A. P., Rosenhouse-Dantsker A., Kowalsky G. B., Logothetis D. E., Levitan I. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8055–8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernier L. P., Ase A. R., Tong X., Hamel E., Blais D., Zhao Q., Logothetis D. E., Séguéla P. (2008) Mol. Pharmacol. 74, 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara Y., Kubo Y. (2006) J. Physiol. 576, 135–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fountain S. J., Parkinson K., Young M. T., Cao L., Thompson C. R., North R. A. (2007) Nature 448, 200–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawate T., Michel J. C., Birdsong W. T., Gouaux E. (2009) Nature 460, 592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.