Abstract
The p110 CUX1 homeodomain protein participates in the activation of DNA replication genes in part by increasing the affinity of E2F factors for the promoters of these genes. CUX1 expression is very weak in quiescent cells and increases during G1. Biochemical activities associated with transcriptional activation by CUX1 are potentiated by post-translational modifications in late G1, notably a proteolytic processing event that generates p110 CUX1. Constitutive expression of p110 CUX1, as observed in some transformed cells, leads to accelerated entry into the S phase. In this study, we investigated the post-translation regulation of CUX1 during mitosis and the early G1 phases of proliferating cells. We observed a major electrophoretic mobility shift and a complete inhibition of DNA binding during mitosis. We show that cyclin B/CDK1 interacts with CUX1 and phosphorylates it at multiple sites. Serine to alanine replacement mutations at 10 SP dipeptide sites were required to restore DNA binding in mitosis. Passage into G1 was associated with the degradation of some p110 CUX1 proteins, and the remaining proteins were gradually dephosphorylated. Indirect immunofluorescence and subfractionation assays using a phospho-specific antibody showed that most of the phosphorylated protein remained in the cytoplasm, whereas the dephosphorylated protein was preferentially located in the nucleus. Globally, our results indicate that the hyperphosphorylation of CUX1 by cyclin B/CDK1 inhibits its DNA binding activity in mitosis and interferes with its nuclear localization following cell division and formation of the nuclear membrane, whereas dephosphorylation and de novo synthesis contribute to gradually restore CUX1 expression and activity in G1.
Keywords: CDK (Cyclin-dependent Kinase), Cyclins, DNA-binding Protein, Mitosis, Transcription, Transcription Factors, CUX1
Introduction
Mitosis in higher eukaryotes is associated with global inhibition of gene transcription (1). Several mechanisms are thought to contribute to this effect, including changes in chromatin structure that restrict accessibility to the DNA template and inhibition of the transcriptional machinery by phosphorylation (reviewed in Ref. 2). Mitotic inhibition of transcription was shown to involve direct phosphorylation of RNA polymerase II and III subunits (3–5). Interestingly, in vitro experiments showed that cyclin B/CDK1 is sufficient to inhibit transcription in reconstituted reactions with purified proteins (4). Phosphorylation was also shown to cause the inhibition or exclusion from the chromatin of a number of gene-specific transcription factors, such as c-Jun, POU, Myc, Myb, Oct1/2, Fos, E2F1, Bcl6, Ets1, and YY1 (6–10). Phosphorylation of transcription factors during mitosis may serve to reset the DNA binding clock allowing for a fresh transcriptional program to begin at the start of each cell cycle. Exclusion from mitotic chromatin, however, is not universal. Indeed, a number of general or specific transcription factors have been shown to remain associated with condensed chromosomes in mitosis, including TFIID (11), TFIIB (11), TATA-binding protein (12), AP-2 (9), p67 SRF (13), heterogeneous nuclear ribonucleoprotein K (14), FBP (14), the RUNX factors (15, 16), NFE2 (17), MYOD (18), C/EBP (9), HSF2 (19), HNF1β (20), and MLL (21). The association of these factors with specific regulatory sequences within mitotic chromosomes was proposed to cooperate with other epigenetic mechanisms, like histone modifications or incorporation of histone variants (22–24), in the marking of genes for rapid regulation following mitosis, a process that was termed bookmarking (14, 25, 26). In particular, both TFIID and HSF2 were found to interact with the PP2A phosphatase and the CAP-G subunit of condensin, thereby promoting dephosphorylation and inactivation of the condensin complex and preventing chromatin compaction in the vicinity (19, 27).
In higher eukaryotic cells, cyclin-dependent kinases and their activating subunits, the cyclins, regulate progression through the cell cycle with cyclin B binding to CDK1 during mitosis (28). Inhibitory phosphorylations by Wee1/Myt1 on Thr14/Tyr15 of CDK1 prevent premature activation during the G2 phase of the cell cycle (29, 30). Activation of cyclin B/CDK1 as cells enter mitosis results from the dephosphorylation of these residues by Cdc25 phosphatases, and the phosphorylation of Thr161 within the T-loop by the cyclin-dependent kinase-activating kinase follows a positive feedback loop whereby cyclin B/CDK1 not only phosphorylates Myt1 and Wee1 to effect their inhibition or degradation but also Cdc25 phosphatases to stimulate their activity. Together these regulatory events lead to an explosion of cyclin B/CDK1 activity (31, 32). A recent proteomics study has identified over 70 proteins that are phosphorylated in mitosis by cyclin B/CDK1, some of which on multiple residues (33). Phosphorylation by cyclin B/CDK1 enables the activation of other major mitotic kinases, such as Plk1 and the Aurora kinases, and regulates the processes involved in nuclear breakdown, spindle assembly, and chromosome condensation (34). Cyclin B/CDK1 eventually contributes to the activation of APC/C, the E3 ubiquitin ligase responsible for the targeted degradation of cyclin B resulting in the inactivation of the kinase complex, an essential step required for chromosome separation and mitotic exit (35). The high degree of connection between these signaling pathways highlights the importance of cyclin B/CDK1. Indeed, a knock-out of the cyclin B1 gene in a mouse model is embryonic lethal (36).
Genetic studies in Drosophila revealed that the Cut transcription factor functions as an important determinant of cell type specificity in multiple organs and tissues (37–39). This function is believed to be conserved in evolution because ectopic expression of the murine or human Cut homeobox protein 1 (CUX1) similarly affected embryonic sensory organ development and was able to rescue the wing scalloping mutant phenotype (40). How Cut or CUX1 would determine cell identity is not known. A number of studies in tissue culture systems have documented the cell cycle-dependent regulation of CUX1. The histone nuclear factor D (HiNF-D), which was later found to include CUX1 as its DNA binding partner, was reported to be up-regulated in S phase in normal cells (41–45). The full-length CUX1 protein binds rapidly but only transiently to DNA (46). The up-regulation of stable DNA binding in the late G1 phase was shown to involve at least two post-translational modifications as follows: dephosphorylation of the Cut homeodomain by the Cdc25A phosphatase and proteolytic cleavage to generate p110 CUX1 (47–49). The activity of the p110 isoform is highest in S phase. Not only is p110 generated at the end of G1 phase, but as cells progress into the G2 phase, DNA binding is down-modulated following phosphorylation by cyclin A/CDK1 of two serine residues in the region of the Cut homeodomain (50). Interestingly, cyclin A/CDK2 interacts with CUX1 during the S phase but does not phosphorylate these residues and, in fact, cooperates with p110 CUX1 in reporter assays (51). In agreement with these findings, constitutive expression of p110 CUX1 accelerates entry into the S phase and stimulates cell proliferation (52). In contrast, the G1 phase is extended in mouse embryo fibroblasts derived from Cux1Z/Z knock-out mice (52).
One proposed function for mitotic bookmarking is to mark those genes that need to be quickly activated in early G1. Another potential function, which does not exclude the first, is the maintenance of cell identity along a given cell lineage. In this context, the molecular bookmark would act as a cell memory mechanism by which cell identity is transmitted to daughter cells. Specific transcription factors involved in bookmarking would be expected to be active not only in mitosis but also in late G2 and early G1. CUX1 is believed to be important for the determination and maintenance of cell identify, but its activity was shown to be weak in early G1 as cells come out of quiescence, the highest in S phase, and gradually weaker in G2 (42, 47–49). In this study, we maintained cells in the presence of growth factors to investigate the regulation of p110 CUX1 from one cell cycle to the next. We report that p110 CUX1 is inactivated in mitosis as the result of hyper-phosphorylation by cyclin B/CDK1. Passage into G1 is associated with the degradation of some p110 CUX1 proteins, whereas the remaining proteins are gradually dephosphorylated and imported into the nucleus. Globally, these results indicate that the hyper-phosphorylation of CUX1 by cyclin B/CDK1 resets CUX1 activity to the zero level at each cell division, although dephosphorylation in G1 reactivates the protein.
EXPERIMENTAL PROCEDURES
Cell Culture
All lines were maintained in Dulbecco's modified minimum essential medium (DMEM) supplemented with penicillin/streptomycin, glutamine, and 10% fetal bovine serum (all from Invitrogen), except for NMuMG cells that were supplemented with 10 μg/ml insulin. Cells were cultured in a humidified incubator at 37 °C.
Generation of Stable Cell Lines
Retroviruses were produced by transfecting 293VSV cells with plasmids encoding CUX1 (Myc-tagged at the N terminus and HA-tagged at the C terminus) inserted in the pREV/TRE vector (Clontech). The supernatant was applied on cells along with 8 μg/ml Polybrene (Roche Applied Science) and plates were centrifuged at 300 × g for 1 h. Stably infected cells were selected for 5 days in hygromycin (pREV/TRE), and at least 500 resistant clones were pooled. Vectors expressing the following proteins were used: p110 CUX1 (amino acids 747–1505); p110 CUX1/TAP tag (amino acids 612–1336-TAP tag). Stable cell lines generated by transfection were produced by co-transfecting CUX1 (amino acids 612–1328 His-tagged N terminus and HA-tagged C terminus) inserted into the pTriEx2 vector (Novagen) and pcDNA3 (Invitrogen) using Lipofectamine 2000 (Invitrogen) following the manufacturers' instructions. Populations were generated by selecting with G418 (Roche Applied Science) for 5 days, and resistant clones were pooled.
Cell Synchronization
Mitotic cells were collected by a gentle shake off after a 10–14-h nocodazole treatment (40–100 ng/ml). To study entry into G1, floating mitotic cells were then washed three times in complete media and re-seeded for 2–16 h.
Microscopy
Cells were grown on glass coverslips and fixed in 3.7% paraformaldehyde. The blocking/solubilization solution consisted in PBS containing 5% FBS and 0.5% Triton X-100. α-HA, α-endogenous-CUX1, and α-CUX1-Ser(P)1237 were used as primary antibodies with Alexa-conjugated, species-specific secondary antibodies (Molecular Probes). DNA was stained with DAPI (Sigma). Confocal images were taken using a Zeiss 510 Meta laser scanning confocal microscope (Carl Zeiss, Canada Ltd, Toronto, Ontario, Canada) with a ×100 objective. Volocity software (PerkinElmer Life Sciences) was used for image analysis.
Fluorescence-activated Cell Sorting (FACS) Analysis
Cells were trypsinized, fixed in 75% EtOH, and stored at −20 °C until analysis. For analysis, 50 μl of FBS was added to each sample. The cells were then centrifuged, washed in PBS, and resuspended in 300 μl of PBS containing 200 μg/ml RNase (Sigma) and 5 μg/ml propidium iodide (Sigma). Samples were incubated for 15 min at 37 °C and analyzed using a FACScan (BD Biosciences), using doublet discrimination to gate single cells. Cell cycle profiles were analyzed with FlowJo (Tree Star software).
Protein Extracts
Nuclear extracts were prepared using a procedure adapted from Lee et al. (53). Briefly, cells were submitted to three freeze/thaw cycles in Buffer A (10 mm Hepes, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 1 mm DTT). Nuclei were then resuspended in Buffer C (20 mm Hepes, pH 7.9, 25% glycerol 1.5 mm MgCl2, 420 mm NaCl2, 0.2 mm EDTA) and incubated at 4 °C for 30 min. After 15 min of centrifugation, the supernatant was collected. Buffers A and C were supplemented with protease and phosphatase inhibitor mix tablet (Roche Applied Science). Total extracts were prepared in RIPA, 0.1% SDS (10 mm Tris-HCl, pH 8, 1 mm EDTA, 0.5 mm EGTA, 150 mm NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 1 mm PMSF, protease and phosphatase inhibitor tablets). After 10 min on ice, the resulting slurry was centrifuged for 15 min at 4 °C, and the supernatant was collected. Mitotic extracts used in kinase assays were prepared from nocodazole-arrested cells. Cells were lysed in 50 mm Tris, pH 7.4, 250 mm NaCl, 1 mm EDTA, 50 nm NaF, 1 mm DTT, 0.1% Triton X-100, 1× protease inhibitor mixture (Roche Applied Science), cleared by centrifugation for 30 min at 15,000 × g, and adjusted to 10 mg/μl.
DNA Binding Assays
EMSAs for exogenous CUX1 were performed with 1–4 μg of total extract from mammalian cells. The samples were incubated at room temperature for 5 min in a final volume of 30 μl of EMSA buffer (25 mm NaCl, 10 mm Tris, pH 7.5, 1 mm MgCl2, 5 mm EDTA, pH 8.0, 5% glycerol, 1 mm of DTT) with 100 ng of poly(dI-dC) and 30 μg of BSA as nonspecific competitors. End-labeled double-stranded oligonucleotides (5′-tcgagacgatatcgataagcttcttttc-3′) were added and further incubated for 15 min at room temperature. Samples were loaded on a 4% polyacrylamide gel (30:1) and separated by electrophoresis at 8 V/cm in Tris-glycine. Gels were dried and visualized by autoradiography. For Southwestern blot analysis, proteins were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were renatured by agitating for 15 min at 4 °C with 6 m guanidine HCl in binding buffer (250 mm Hepes, pH 7.9, 30 mm MgCl, 500 mm KCl, 1 mm DTT). This was repeated using serial dilutions of guanidine HCl in binding buffer down to 0.185 m and finally in binding buffer alone. The membrane was then incubated in blocking buffer (binding buffer, 5% milk, phosphatase inhibitors (Roche Applied Science)) for 2 h at room temperature. Hybridization was carried out at 4 °C overnight, using end-labeled double-stranded oligonucleotides (5′-tcgagacgatatcgataagcttcttttc-3′) diluted in binding buffer plus 1% milk and phosphatase inhibitors. The membrane was then washed three times in binding buffer, dried, and exposed to autoradiography.
Immunoblotting
Proteins extracts were resuspended in Laemmli buffer, boiled for 5 min, resolved by SDS-PAGE, and electrophoretically transferred to a PVDF membrane. Membranes were blocked in TBS-T (10 mm Tris, pH 8, 150 mm NaCl, 0.1% Tween X-100) containing 5% milk and 2.5% bovine serum albumin. Membranes were then incubated with primary antibodies diluted in TBS-T, washed in TBS-T, and incubated with species-specific secondary antibodies conjugated to horseradish peroxidase for 40 min at room temperature. Proteins were then visualized using the ECL system of Amersham Biosciences according to the manufacturer's instructions. The following antibodies and dilutions were used: CUX1 (α861, 1:2000; α1300, 1:2000 (47)), γ-tubulin (1:15,000, Sigma), HA (1:2000, MMS-101R, Covance), CDK1 (1:700, Ab-4, NeoMarkers), and cyclin B1 (1:500, Neomarkers).
Kinase Assay
Lysates from Sf9 cells expressing cyclin B/CDK1 (Fig. 2), mitotic extracts, and purified PKA (Sigma) (supplemental Fig. S1) were used as sources of kinase. His-purified CUX1 recombinant proteins were incubated with 5 μCi of [γ-32P]ATP (6000 Ci/mmol) (Amersham Biosciences) in kinase buffer (50 mm Hepes, pH 7.5, 10 mm MgCl2, 1 mm DTT, 1 mm NaF). Reactions were allowed to proceed for 10–20 min at 30 °C and were terminated by adding 6 μl of SDS loading buffer and boiling for 5 min. Proteins were resolved on 6% or 10% SDS-PAGE, dried, and exposed to x-ray films.
FIGURE 2.
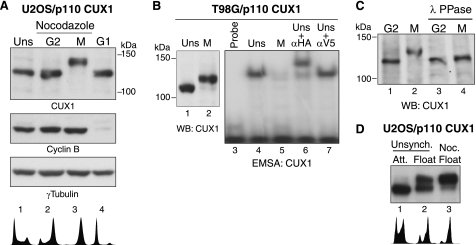
DNA binding activity of recombinant p110 CUX1 is inhibited during mitosis. A, U2OS/p110 CUX1 cells were treated with nocodazole for 12 h. Mitotic floating cells were collected (M, lane 3) and replated in nocodazole-free media for 2 h (G1, lane 4), and attached cells (G2, lane 2) were collected separately. Immunoblotting was performed using CUX1, cyclin B, or γ-tubulin antibodies as indicated. B, DNA binding activity of HA-tagged p110 CUX1 from unsynchronized (UNS) or nocodazole-arrested mitotic cells (M) was analyzed by EMSA. The supershift in the presence of HA antibodies confirmed the identity of the p110 CUX1 retarded complex, whereas an unrelated antibody (αV5) had no effect. C, G2 and mitotic cells lysates from U2OS/p110 CUX1 cells were treated with λ-phosphatase (λPPase) for 30 min and subjected to SDS-PAGE and immunoblotting. D, immunoblotting using a CUX1 antibody was performed using whole cell extracts from unsynchronized (Unsynch.) cells, cells collected by mitotic shake-off from an unsynchronized population, and nocodazole (Noc.)-arrested mitotic cells. WB, Western blot; Att, attached.
Phosphoamino Acid Analysis
Bacterially purified CUX1 (amino acids 612–1328) was phosphorylated using lysates from Sf9 cells expressing cyclin B/CDK1 complex. Phosphorylated proteins were separated by SDS-PAGE and electrophoretically transferred to PVDF membrane. The membrane was washed in a large volume of water, dried, and rewetted with methanol and water and dried again. Membrane was exposed to x-ray film to visualize the phosphorylated species and cut out the membrane. Phosphoamino acid analysis was performed by hydrolysis of the membrane-bound proteins in constantly boiling 6 n HCl for 1 h at 100 °C. The resulting amino acids were dried, resuspended in 5 μl of pH 1.9 buffer (0.58 m formic acid, 1.36 m glacial acetic acid), and applied to TLC plates. Separation was achieved by two-dimensional electrophoresis, first in pH 1.9 buffer for 1 h at 500 V and then in pH 3.5 buffer (0.87 m glacial acetic acid, 0.5% pyridine (v/v), 0.5 mm EDTA) for 1 h at 500 V. 1 μl of nonradioactive phosphoamino acid standard mixture containing phosphoserine, phosphothreonine, and phosphotyrosine (10 mg/ml) were spotted on top of each sample. Following electrophoresis, the plate was dried for 20 min and sprayed with ninhydrin to indicate the position of single amino acids, and the phosphorylated amino acids were revealed by autoradiography.
RESULTS
CUX1 DNA Binding Activity Is Inhibited in Mitosis
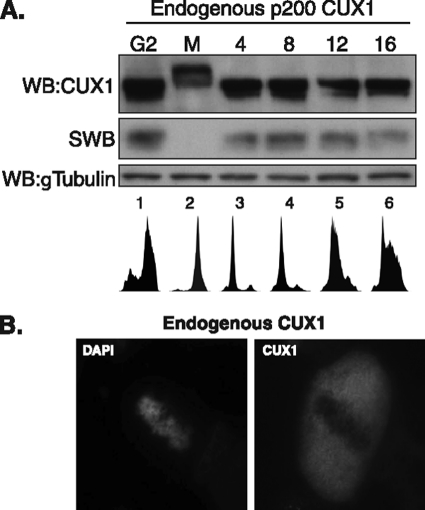
We initially observed that CUX1 proteins from cells in mitosis migrated more slowly in SDS-PAGE (Fig. 1A). This was seen in mitotic cells collected by shake off after treatment with nocodazole (Fig. 1A, lane 2), whereas the attached cells in the same dish, which are mostly cells progressing through G2, displayed CUX1 proteins of lower apparent molecular weight (Fig. 1A, lane 1). Interestingly, the normal CUX1 migration pattern was restored when mitotic cells were relieved of the nocodazole block by re-plating into fresh medium (Fig. 1A, lanes 3–6). The slower electrophoretic mobility of CUX1 in mitosis was associated with the inhibition of DNA binding, as determined by Southwestern blot analysis (Fig. 1A, middle panel). In agreement with this result, indirect immunofluorescence using an asynchronous population of cells revealed that CUX1 was dissociated from the chromatin during mitosis (Fig. 1B). These results indicate that CUX1 DNA binding activity is inhibited in mitosis, and this correlates with post-translational modifications that reduce its electrophoretic mobility.
FIGURE 1.
DNA binding activity of endogenous p200 CUX1 is inhibited during mitosis. A, U2OS cells were treated with nocodazole (40 ng/ml) for 12 h. Floating mitotic cells were collected (M, lane 2) and replated in nocodazole-free media for 4–16 h (lanes 3–6). Whole cells lysates were subjected to SDS-PAGE, followed by immunoblotting using CUX1 antibodies (top panel) or Southwestern (SWB) analysis using a radiolabeled CUX1 probe (middle panel). Immunoblotting for γ-tubulin was used as loading control (bottom panel). WB, Western blot. B, CUX1 localization in mitotic cells was visualized by indirect immunofluorescence confocal microscopy. Asynchronous NMuMG cells were fixed and stained using CUX1 antibodies and DAPI. Mitotic cells were identified based on chromosome condensation.
Exogenous p110 CUX1 Is Hyperphosphorylated and Its DNA Binding Is Inhibited during Mitosis
We next investigated whether short CUX1 isoforms such as p110 CUX1 displayed a similar regulation during mitosis. A recombinant p110 CUX1 protein (Myc/747-1505/HA) was expressed by retroviral transduction in multiple cell lines. In all cell lines tested, p110 CUX1 displayed a slower migration in SDS-PAGE when lysates were prepared from mitotic nocodazole-arrested cells (Fig. 2, A, lane 3, and B, lane 2; and data not shown). Slower migration of CUX1 again correlated with a drastic inhibition of p110 CUX1 DNA binding activity as judged from electrophoretic mobility shift assays (Fig. 2B, compare lanes 4 and 5). The electrophoretic mobility shift seemed primarily due to phosphorylation because treatment of a mitotic lysate with λ-phosphatase restored migration in SDS-PAGE (Fig. 2C, compare lanes 2 and 4). Importantly, a similar shift in migration was observed when mitotic cells were collected from an asynchronous cell population (Fig. 2D, compare lanes 2 and 3). We conclude that p110 CUX1 contains the amino acid residues that are post-translationally modified during mitosis and that this regulation of CUX1 is not simply caused by acute activation of the spindle assembly checkpoint in response to nocodazole treatment but is linked to the passage through mitosis.
Cyclin B/CDK1 Phosphorylates CUX1 on Serines 1237 and 1270
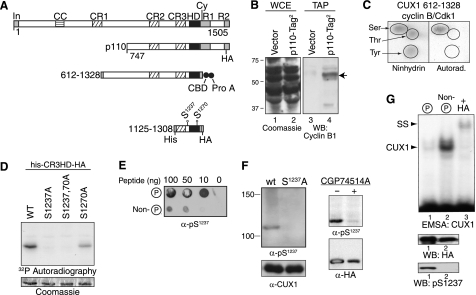
We reported previously that cyclin A/CDK1 phosphorylates CUX1 on serines 1237 and 1270 and that phosphorylation at these positions inhibit CUX1 DNA binding activity (50). We hypothesized that cyclin B/CDK1 may further phosphorylate serine 1237 upon entry in mitosis. We first verified whether CUX1 and cyclin B1 interact with each other. We performed tandem affinity purification using cells expressing a recombinant p110 CUX1 protein with two tags at its C terminus. Cyclin B1 co-purified with CUX1-Tag2 but was not detected when the same procedure was carried in parallel using cells carrying the empty vector (Fig. 3B, lanes 3 and 4). We then performed in vitro kinase reactions using a preparation of cyclin B/CDK1 from Sf9 insect cells and a bacterially expressed His-tagged CUX1 protein containing amino acids 612–1328. Phosphoamino acid analysis revealed that phosphorylation of CUX1 occurred mainly on serine residues (Fig. 3C). Serine to alanine substitutions in a smaller CUX1 protein encompassing the Cut repeat 3 and the homeodomain (CR3HD) confirmed that serine 1237 is a major site of phosphorylation within this region, although serine 1270 either is weakly phosphorylated or has a mild effect on the phosphorylation of serine 1237 (Fig. 3D). We raised CUX1-Ser(P)1237 phosphospecific antibodies and verified their specificity using multiple approaches as follows: dot blot assays using phosphorylated and nonphosphorylated peptides (Fig. 3E); immunoblotting of recombinant p110 CUX1 carrying a serine (WT) or an alanine residue at position 1237 (Fig. 3F, left panel); and immunoblotting of wild type recombinant p110 CUX1 following treatment of cells with the CDK1 inhibitor CGP74514A (Fig. 3F, right panel). To evaluate the effect of phosphorylation by cyclin B/CDK1 on the DNA binding activity of CUX1, the kinase reaction was repeated in the presence of cold ATP and the phosphorylated and nonphosphorylated CUX1 proteins were separated by affinity chromatography using the Qiagen PhosphoProtein purification kit. Immunoblotting using Ser(P)1237-specific antibodies confirmed that the proteins were well separated (Fig. 3G, bottom panel). Although immunoblotting with the HA antibodies indicated that the phosphorylated protein was more abundant than the nonphosphorylated one, in EMSA the retarded complex was much more abundant with the nonphosphorylated protein. Together, these results demonstrate that cyclin B/CDK1 interacts with CUX1, phosphorylates it on serine 1237, and inhibits its DNA binding activity.
FIGURE 3.
Cyclin B/CDK1 phosphorylates CUX1 on serines 1237 and 1270. A, diagrammatic representation of CUX1 and the recombinant proteins used to investigate phosphorylation by cyclin B/CDK1. Shown at the top are the functional domains and the cyclin-interacting motif. In, inhibitory domain; CC, coiled-coil; CR1, CR2, and CR3, cut repeats 1, 2, and 3; HD, cut homeodomain; Cy, cyclin-binding motif. Epitope tags are as follows: CBD, calmodulin binding domain; Prot A, protein A; His, histidine tag; HA, hemagglutinin. B, interaction between p110 CUX1 and cyclin B1 was investigated in cells expressing a recombinant p110 CUX1 protein with two tags at its C terminus, p110-Tag2. Whole cell extracts (WCE) were separated by SDS-PAGE and stained with Coomassie Blue (left panel) or were submitted to tandem affinity purification and analyzed by immunoblotting using a cyclin B1 antibody (right). As a control, the same procedures were performed using cells carrying the empty vector (vector). C, recombinant His-tagged CUX1 protein containing amino acids 612–1328 was purified from bacteria and then phosphorylated in vitro using [γ-32P]ATP and a preparation of cyclin B/CDK1 from Sf9 cells. Kinase reactions were subjected to acid hydrolysis, mixed with an excess of phosphoamino acid (serine, threonine, and tyrosine), and subjected to two-dimensional electrophoresis on TLC plates. The position of single amino acids was revealed with ninhydrin, and phosphorylated CUX1 amino acids were revealed by autoradiography. D, in vitro kinase assays in the presence of [γ-32P]ATP were performed using cyclin B/CDK1 from Sf9 cells and recombinant CUX1 proteins containing the cut repeat 3 and Cut homeodomain (CR3-HD), a histidine, and a hemagglutinin tag (his-CR3HD-HA, CUX1 amino acids 1125–1308) and carrying serine to alanine substitutions at residues 1237 and 1270, as indicated. Protein loading was verified by Coomassie Blue staining and phosphorylation, by autoradiography. E, rabbits were immunized with a phosphopeptide (Cys-YSQGApSPQPQHQ) corresponding to serine 1237 and its surrounding residues. Dot blot assays were performed to compare the affinity of unpurified bleeds for phospho- and nonphosphorylated peptides. F, immunoblotting was performed with purified anti-Ser(P)1237 and anti-CUX1 antibodies. Left, whole cell extracts from NMuMG cells stably expressing either wild type (wt) p110 CUX1 or p110S1237,1270A CUX1 (S1237A). Right, whole cell extracts from HEK293 cells transiently transfected with p110 CUX1-HA and treated or not for 16 h with the CDK1 inhibitor CGP74514A (20 μm). G, kinase assays were performed as detailed in C but with cold ATP. Phosphorylated and nonphosphorylated proteins were separated by affinity chromatography using the Qiagen phosphoprotein purification column and separated by SDS-PAGE. Phosphorylated and nonphosphorylated CUX1 proteins were analyzed by immunoblotting with an α-HA antibody and a phosphoserine 1237-specific antibody, and in EMSA using a using a radiolabeled CUX1 probe. The supershift (SS) in the presence of HA antibodies confirmed the identity of the CUX1 retarded complex (lane 3). WB, Western blot.
CDK1 and PKA Phosphorylate CUX1 in Mitosis
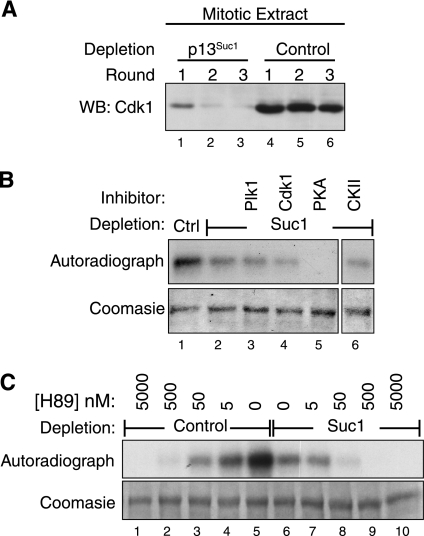
As an approach to investigate whether kinases other than cyclin B/CDK1 phosphorylate CUX1 in mitosis, we performed in vitro kinase assays using bacterially expressed his-CUX1(612–1328) as a substrate and mitotic extracts as a source of kinase. To eliminate the signal due to phosphorylation by cyclin B/CDK1, the extracts were first depleted by affinity chromatography using beads coupled to p13Suc1, a protein related to human CKS that has high affinity for cyclin-dependent kinases. Immunoblotting assays confirmed that the extract was essentially devoid of CDK1 after three rounds of depletion (Fig. 4A, compare lanes 3 and 6). Mitotic extracts corresponding to Fig. 4A, lanes 3 and 6, were used in subsequent kinase assays. Depletion of CDK1 from the mitotic extract reduced, but did not eliminate, the phosphorylation of CUX1 (Fig. 4B, lanes 1 and 2). This result confirms that other kinases in the mitotic extract can phosphorylate CUX1. We then performed kinase assays using the CDK1-depleted extract in the presence of excess inhibitors for Plk1, CDK1, PKA, or CKII. A significant reduction in the level of phosphorylation was observed only in the presence of the PKA inhibitor, H89 (Fig. 4B, lane 5). Importantly, inhibition of phosphorylation in the mitotic extract was also observed with lower concentrations of H89 that were unable to inhibit cyclin B/CDK1. Indeed, 50 nm H89 was sufficient to annihilate phosphorylation using the CDK1-depleted extract (Fig. 4C, lane 8), whereas the same concentration did not eliminate phosphorylation in the complete mitotic extract that contains cyclin B/CDK1 (Fig. 4C, lane 3), nor was it able to reduce significantly the phosphorylation of CUX1 using a preparation of cyclin B/CDK1 from Sf9 insect cells (data not shown). In summary, the depletion of CDK1 from mitotic extracts confirmed that cyclin B/CDK1 plays a major role in the phosphorylation of CUX1 in mitosis, whereas kinase assays in the presence of kinase inhibitors pointed to PKA as another kinase that can phosphorylate CUX1 in mitosis.
FIGURE 4.
PKA and CDK1 contribute to CUX1 phosphorylation in mitosis. A, mitotic cells lysates from nocodazole-arrested T98G cells were prepared in nondenaturing conditions. CDK1 was depleted from the extract using three successive incubations with p13Suc1-agarose beads or beads alone as a control. Immunoblotting was done using CDK1 antibodies. Lysates corresponding to lanes 3 and 6 were used as a source of kinase in subsequent experiments. WB, Western blot. B, His-CUX1(612–1328) was purified from bacteria and used as a substrate in kinase assays using, as a source of kinases, either control or CDK1-depleted lysates (lanes 1 and 2, respectively). Reactions were done in parallel in the presence of inhibitors against PLK1 (500 nm BI2536), CDK1 (2.5 μm CGP74514A), PKA (20 μm H89), or CKII (60 μm 4,5,6,7-tetrabromobenzotriazole), as indicated. C, kinase reactions were performed as in B, in the presence of various concentrations of the PKA inhibitor H89. WB, Western blot.
Phosphorylation by Cyclin B/CDK1 Is Responsible for the Inhibition of CUX1 DNA Binding in Mitosis
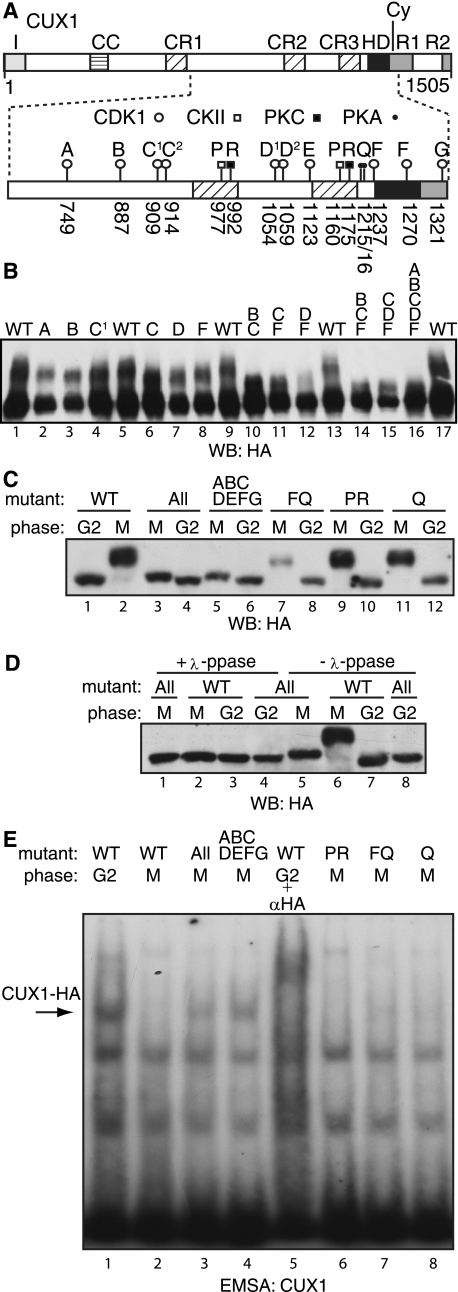
Serine to alanine replacement mutations at SP dipeptide sites were engineered to identify the phosphorylation site(s) responsible for the change in electrophoretic mobility and the inhibition of DNA binding in mitotic cells (Fig. 5A). A panel of mutants was tested by transient transfection in U2OS cells (Fig. 5B). Because transfected cells express the recombinant protein in excess, one can observe the unphosphorylated species in addition to the supershifted bands that appear as a smear (Fig. 5B). Mutations at each single site affected the mobility, yet no combination of mutations was able to completely eliminate the supershift (Fig. 5B). We therefore generated a second series of mutants which, in addition to all SP dipeptide sites, carried replacement mutations at all previously identified phosphorylation sites in this region of CUX1. These include CKII and PKC sites in Cut repeats 2 and 3 and PKA sites in the linker between Cut repeat 3 and the Cut homeodomain (Fig. 5A) (54–56). Populations of U2OS stably carrying the expression vectors were generated and then synchronized in G2 or M before testing the recombinant proteins in immunoblotting and EMSA. Replacement mutations at either the PKA sites (Q mutant), the CKII and PKC sites (PR mutant), or the PKA sites plus the cyclin B/CDK1 sites at serine 1237 and 1270 (FQ mutant) did not appreciably affect the supershift and did not restore DNA binding (Fig. 5, C and E). However, the replacement of all SP dipeptide sites greatly reduced the supershift and importantly was sufficient to restore CUX1 DNA binding in mitosis (ABCDEFG mutant: Fig. 5, C, lanes 5 and 6, E, lane 4). The slight supershift observed in mitosis persisted when replacement mutations were made at all 16 sites (All mutant, Fig. 5C, lanes 3 and 4). This supershift probably results from the phosphorylation at yet another site because it disappears upon treatment with λ-phosphatase (Fig. 5D, lanes 1 and 5). Although we did not observe the phosphorylation of threonine residues by cyclin B/CDK1 in vitro (Fig. 3C), we speculate that one or more of the four TP dipeptide sites present in this region of CUX1 might be phosphorylated in vivo. Nevertheless, phosphorylation at these sites is not responsible for the inhibition of DNA binding because the ABCDEFG mutant was able to bind to DNA in mitosis (Fig. 5E, lane 4).
FIGURE 5.
CUX1 is phosphorylated at several SP dipeptide sites in mitosis. A, diagrammatic representation of the recombinant CUX1(612–1328/HA) protein showing the phosphorylation sites for PKA (Ser1215 and Ser1216), CKII (Ser977 and Ser1160), PKC (Thr992 and Ser1175), and the SP dipeptide sites that could be phosphorylated by CDK1 (Ser749, Ser887, Ser909, Ser914, Ser1054, Ser1059, Ser1123, Ser1237, Ser1270, and Ser1321). Alanine replacement mutations are indicated by letters. B, recombinant CUX1(612–1328/HA) proteins carrying alanine replacement mutations were expressed by transient transfections in U2OS cells and analyzed by immunoblotting. The letters above each lane indicate the alanine replacement mutations within the protein: WT (wild type), A (749), B (887), C1 (909), C (909, 914), D (1054, 1059), F (1237, 1270), and G (1321). C–E, populations of U2OS cells stably expressing recombinant CUX1(612–1328/HA) proteins were synchronized in G2 or M phase as described in Fig. 2A and analyzed by immunoblotting and EMSA. Where indicated, protein extracts were first incubated in the presence of λ-phosphatase (λ-ppase). The letters above each lane indicate the alanine replacement mutations within the protein, as described in A: WT (wild type), All (all SP dipeptides, CKII, PKC, PKA: 749, 887, 909, 914, 977, 992,1054,1059,1123, 1160,1175, S1215,1216, 1237,1270,1321), ABCDEFG (all SP dipeptides: 749, 887, 909, 914, 1054, 1059, 1123, 1237, 1270, 1321), FQ (1215,1216,1237,1270), PR (CKII+PKC: 977, 992, 1160, 1175), Q (PKA, 1215, 1216).
Dephosphorylation and de Novo Protein Synthesis Slowly Restore CUX1 DNA Binding in G1
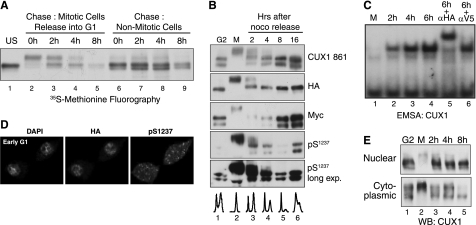
We performed in vivo labeling and pulse-chase analysis to investigate the fate of CUX1 after mitosis and compare its stability in G2, mitosis, and early G1. T98G cells expressing p110 CUX1 cells were incubated with [35S]methionine and nocodazole for 10 h, at which point (the 0-h time point in Fig. 6A) mitotic cells that were floating were isolated and replated in fresh medium, and nonmitotic cells that remained attached were re-fed with fresh medium. Two main observations can be made from this analysis. First, the supershifted CUX1 species gradually disappeared to be replaced by faster migrating species (Fig. 6A, compare lane 2 with lane 4). Second, a slight reduction in CUX1 levels between M and G1 was apparent (Fig. 6A, compare lane 2 with lane 4), and CUX1 was stable in nonmitotic cells over the same period of time (Fig. 6A, lanes 6–8). These results suggest that mitotic exit is associated with degradation of some CUX1 proteins and dephosphorylation of remaining CUX1 proteins. In parallel, we analyzed the steady-state level of CUX1 by performing immunoblotting with antibodies against CUX1 (861), the N-terminal tag (Myc), the C-terminal tag (HA), and phosphoserine 1237 (Fig. 6B). We noticed that expression of CUX1 in T98G cells generates a doublet of bands (Fig. 6A). To investigate the reason for this, we extended the time of migration to better separate the bands. Two bands were observed with all antibodies except the Myc antibody that recognizes the N-terminal tag, indicating that the lower band is the product of proteolytic processing at the C terminus, at a site downstream of the previously characterized cleavage site (Fig. 6B and data not shown) (57). This proteolytic processing was not observed in other cell lines and was not further characterized. The appearance of the supershifted species in mitosis correlated with the phosphorylation of CUX1 at serine 1237, which is phosphorylated by cyclin B/CDK1 (Fig. 6B, lanes 1 and 2). The transition from mitosis to early G1 and the progression in G1 was accompanied by a reduction in the amount of phosphorylated CUX1 protein, as judged from immunoblotting with the Ser(P)1237 phosphospecific antibodies (Fig. 6B, compare lanes 2–5) and a corresponding increase in CUX1 DNA binding activity as judged from EMSA analysis (Fig. 6C, lanes 1–4). This result together with the transition from slow to fast migrating species observed in the pulse-chase assay (Fig. 6A) suggests that some CUX1 proteins are dephosphorylated in early G1. Indirect immunofluorescence performed 4 h after replating mitotic cells in the absence of nocodazole showed that the bulk of CUX1 protein was present within the nucleus (Fig. 6D, HA). In contrast, the signal obtained with the phosphospecific Ser(P)1237 antibody was present within the entire cell, suggesting that phosphorylated CUX1 proteins may not be as efficiently partitioned into the nucleus (Fig. 6D, pS1237). Subcellular fractionation followed by immunoblotting confirmed most of the fast migrating CUX1 proteins were present in the nuclear fraction at 2, 4, and 8 h after mitosis, and the slow migrating species remained in the cytoplasmic fraction (Fig. 6E).
FIGURE 6.
Phosphorylation-dephosphorylation restores low CUX1 DNA binding in G1. A, pulse-chase analysis in T98G cells expressing a recombinant p110 CUX1 protein (Myc/747-1505/HA). Cells were incubated for 10 h in the presence of nocodazole and [35S]methionine; floating (Mitotic) and attached (Nonmitotic) cells were separated and re-fed with fresh medium. At the indicated time points, whole cell extracts were prepared and submitted to immunoprecipitation with anti-CUX1 antibodies; proteins were separated by SDS-PAGE and revealed by fluorography. Only attached cells were collected for lanes 6–9 to look at CUX1 stability in nonmitotic cells. B, T98G cells expressing p110 CUX1(Myc/747-1505/HA) were treated with nocodazole for 12 h; attached (G2) and floating (M) cells were separated, and the floating cells were replated in fresh medium; at the indicated time, whole cell extracts were prepared and analyzed by immunoblotting with the indicated antibodies using amounts of protein lysates as specified in B. C, U2OS cells expressing p110 CUX1(Myc/747-1505/HA) were treated with nocodazole for 12 h, and the floating cells were replated in fresh medium. At the indicated time points, extracts were prepared and analyzed in EMSA using a CUX1 probe, as described in Fig. 2B. D, cells were prepared as in C but were replated on a cover slide, incubated for 4 h, and then stained with DAPI and analyzed by indirect immunofluorescence using HA and Ser(P)1237-specific antibodies. E, T98G expressing p110 CUX1 was treated as described in C, and subcellular fractionations were performed to obtain crude cytoplasmic and nuclear extracts, which were analyzed by immunoblotting using anti-CUX1 antibodies.
DISCUSSION
Bookmarking of specific genes in mitosis was proposed to serve as a cell memory mechanism by which cell identity is transmitted to daughter cells (18, 26). The phenotypes conferred by mutations in the cut locus in Drosophila and their rescue by the human and murine CUX1 proteins have implicated CUX1 as a determinant of cell type specificity (37–40). In this study, we present evidence to show that CUX1 is completely inactivated during mitosis as a result of hyperphosphorylation by cyclin B/CDK1. These results clearly exclude that might play a role in mitosis. Whatever the mechanism by which CUX1 determines cell fate, it does not involve bookmarking.
Phosphorylation of serines 1237 and 1270 by cyclin A/CDK1 was previously shown to partially inhibit DNA binding by CUX1 during the G2 phase of the cell cycle (50). Using a small CUX1 peptide consisting of CR3HD, we confirmed that these sites are also targeted by the cyclin B-CDK1 kinase complex. However, using a larger CUX1 peptide containing amino acids 612–1328, we found that other sites were also phosphorylated by cyclin B/CDK1 because a slower migrating species was still produced in mitosis following serine to alanine replacement mutations at Ser1237 and Ser1270 (Fig. 5, B, lane 8, and C, lane 7). Moreover, these mutations did not prevent the inhibition of DNA binding in mitosis (Fig. 5E, lane 7). Mutation of all 10 SP dipeptide sites was required to restore DNA binding activity in mitosis (Fig. 5E, lane 4) and to reduce to a minimum the electrophoretic shift caused by phosphorylation (Fig. 5C, lane 5). The residual shift in this mutant was still due to phosphorylation as it was abolished after treatment with λ-phosphatase (Fig. 5D, lane 1). This shift was not caused by phosphorylation from any of the other known CUX1 kinases, CKII, PKC, or PKA, as mutations at the corresponding sites had no effect. We consider it likely that the residual shift was due to phosphorylation by cyclin B/CDK1 at one or more of the four TP dipeptide sites in this region of CUX1. We did not detect threonine phosphorylation in the phosphoamino acid analysis (Fig. 3C), but a weak signal could have been masked by the abundance of serine phosphorylation. Indeed cyclin B/CDK1 is very promiscuous kinase during mitosis and is known to phosphorylate a wide variety of substrates regulating many different processes in the cell (33, 58). A striking observation of a recent phospho-proteomic study was that over 280 proteins were found to be phosphorylated on more than 10 sites during mitosis (58). Incidentally, CUX1 was reported to be phosphorylated on two SP dipeptide sites in this study, and our own analysis has revealed over 10 phosphorylation sites in CUX1. This would suggest that the number of heavily phosphorylated proteins in mitosis might have been underestimated. Together, these data clearly illustrate the extent and the excess of cyclin B/CDK1 activity in mitosis.
The effect of phosphorylation by cyclin B/CDK1 raises interesting questions with regard to the mechanism by which p110 CUX1 binds to DNA. In general, phosphorylation within a DNA binding domain has an inhibitory effect due to the electrostatic repulsion resulting from the addition of a negative charge. This effect is not observed when phosphorylation occurs outside of the DNA binding domain. On the contrary, phosphorylation at a distance can even stimulate DNA binding as a result of a conformational change that unmasks the DNA binding domain (59). In the case of CUX1, most SP dipeptide sites are located in the linker region and at a distance from the DNA binding domains: cut repeat 2 (CR2), cut repeat 3 (CR3), and the homeodomain (HD) (Fig. 5A). Replacement mutations at the three sites that are close to a DNA binding domain was not sufficient to restore DNA binding in mitosis (data not shown). In a previous study, DNA binding assays with various combinations of domains suggested the Cut homeodomain interacts and cooperates with either CR2 or CR3 but not both at the same time (46). Based on these observations, we consider it likely that phosphorylation of serine residues in the linker regions may alter the conformation of the protein and prevent the proper alignment of the Cut homeodomain with either of the cut repeats, thus causing inhibition of DNA binding.
We noted that CUX1 DNA binding activity was restored in mitosis following serine to alanine replacement mutations at all SP dipeptide sites, despite the fact that this mutant may have been phosphorylated by PKA in mitosis (Fig. 5E). This result was surprising in light of a previous study reporting the inhibition of CUX1 activity by PKA (56). This led us to test directly the effect of PKA phosphorylation on CUX1 DNA binding activity in vitro. Our results from in vitro kinase assays revealed that although phosphorylation by PKA can inhibit DNA binding by a CUX1 protein containing only CR3HD, it did not affect the activity of a longer protein that contains the entire C-terminal region (supplemental Fig. S1). This finding helps explain why the ABCDEF mutant CUX1 protein that can be phosphorylated by PKA during mitosis was able to bind to DNA and suggests that the effect of PKA inhibitors on CUX1 activity in cells may be more complex than originally thought.
One limitation of our study is that the recombinant p110 CUX1 protein was expressed under the control of a heterologous promoter that is constitutive throughout the cell cycle. Therefore, it is important to stress that our study pertains only to some aspect of post-translational regulation in mitosis and early G1. Moreover, we cannot exclude that regulation might have been affected by the fact that the recombinant protein was overexpressed. For example, we showed that a fraction of the protein was degraded in mitosis, and the remaining fraction gradually regained the capacity to bind to DNA as it was dephosphorylated in early G1 (Fig. 6, A–C and E). It is likely that the degradation that takes place in mitosis leaves a smaller fraction of endogenous p110 CUX1 protein. Another aspect of regulation concerns the increase in DNA binding that results from the de novo synthesis of p110 CUX1 in early G1. Although in normal cells the proteolytic processing event that generates p110 CUX1 does not take place until late G1 (47, 49), this element of regulation is lost in many transformed cells, and p110 CUX1 is detectable in the early G1 fraction purified by centrifugal elutriation (60). Expression of a recombinant p110 CUX1 protein from a retroviral vector therefore mimics the situation in transformed cells, although it reaches much higher steady-state levels. Yet, despite the artificially excessive levels of p110 CUX1 in our experimental system, DNA binding was completely inhibited in mitosis as a result of hyperphosphorylation and only gradually reappeared in early G1 (Fig. 6C). Moreover, a large fraction of the phosphorylated proteins remained in the cytoplasm during this period (Fig. 6E). Together these observations provide another illustration of the high levels of cyclin B/CDK1 activity in mitosis and establish the importance of post-translational modifications in the regulation of p110 CUX1 during mitosis and early G1.
Supplementary Material
Acknowledgments
We are grateful to Dr. Robert P. Fisher, Dr. David O. Morgan, and Dr. Stéphane Larochelle for the generous gift of baculovirus vectors expressing Cdk1-HA, Cdk7-HA, and cyclin B. We acknowledge the expert technical assistance of Ginette Bérubé.
Footnotes
This work was supported in part by Grant 019389 from the Canadian Cancer Society (to A. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
REFERENCES
- 1.Prescott D. M., Bender M. A. (1962) Exp. Cell Res. 26, 260–268 [DOI] [PubMed] [Google Scholar]
- 2.Gottesfeld J. M., Forbes D. J. (1997) Trends Biochem. Sci. 22, 197–202 [DOI] [PubMed] [Google Scholar]
- 3.Segil N., Guermah M., Hoffmann A., Roeder R. G., Heintz N. (1996) Genes Dev. 10, 2389–2400 [DOI] [PubMed] [Google Scholar]
- 4.Long J. J., Leresche A., Kriwacki R. W., Gottesfeld J. M. (1998) Mol. Cell. Biol. 18, 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesfeld J. M., Wolf V. J., Dang T., Forbes D. J., Hartl P. (1994) Science 263, 81–84 [DOI] [PubMed] [Google Scholar]
- 6.Segil N., Roberts S. B., Heintz N. (1991) Science 254, 1814–1816 [DOI] [PubMed] [Google Scholar]
- 7.Caelles C., Hennemann H., Karin M. (1995) Mol. Cell. Biol. 15, 6694–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lüscher B., Eisenman R. N. (1992) J. Cell Biol. 118, 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Balbás M. A., Dey A., Rabindran S. K., Ozato K., Wu C. (1995) Cell 83, 29–38 [DOI] [PubMed] [Google Scholar]
- 10.Rizkallah R., Hurt M. M. (2009) Mol. Biol. Cell 20, 4766–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christova R., Oelgeschläger T. (2002) Nat. Cell Biol. 4, 79–82 [DOI] [PubMed] [Google Scholar]
- 12.Chen D., Hinkley C. S., Henry R. W., Huang S. (2002) Mol. Biol. Cell 13, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier-Rouvière C., Cavadore J. C., Blanchard J. M., Lamb N. J., Fernandez A. (1991) Cell. Regul. 2, 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelotti E. F., Sanford S., Levens D. (1997) Nature 388, 895–899 [DOI] [PubMed] [Google Scholar]
- 15.Young D. W., Hassan M. Q., Pratap J., Galindo M., Zaidi S. K., Lee S. H., Yang X., Xie R., Javed A., Underwood J. M., Furcinitti P., Imbalzano A. N., Penman S., Nickerson J. A., Montecino M. A., Lian J. B., Stein J. L., van Wijnen A. J., Stein G. S. (2007) Nature 445, 442–446 [DOI] [PubMed] [Google Scholar]
- 16.Ali S. A., Zaidi S. K., Dobson J. R., Shakoori A. R., Lian J. B., Stein J. L., van Wijnen A. J., Stein G. S. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 4165–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin L., Zhou G. L., Song W., Wu X. S., Wei G. H., Hao D. L., Lv X., Liu D. P., Liang C. C. (2007) BMC Mol. Biol. 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S. A., Zaidi S. K., Dacwag C. S., Salma N., Young D. W., Shakoori A. R., Montecino M. A., Lian J. B., van Wijnen A. J., Imbalzano A. N., Stein G. S., Stein J. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6632–6637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing H., Wilkerson D. C., Mayhew C. N., Lubert E. J., Skaggs H. S., Goodson M. L., Hong Y., Park-Sarge O. K., Sarge K. D. (2005) Science 307, 421–423 [DOI] [PubMed] [Google Scholar]
- 20.Verdeguer F., Le Corre S., Fischer E., Callens C., Garbay S., Doyen A., Igarashi P., Terzi F., Pontoglio M. (2010) Nat. Med. 16, 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blobel G. A., Kadauke S., Wang E., Lau A. W., Zuber J., Chou M. M., Vakoc C. R. (2009) Mol. Cell 36, 970–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow C. M., Georgiou A., Szutorisz H., Maia e Silva A., Pombo A., Barahona I., Dargelos E., Canzonetta C., Dillon N. (2005) EMBO Rep. 6, 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouskouti A., Talianidis I. (2004) EMBO J. 24, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valls E., Sanchez-Molina S., Martinez- Ä M. A. (2005) J. Biol. Chem. 280, 42592–42600 [DOI] [PubMed] [Google Scholar]
- 25.Sarge K. D., Park-Sarge O. K. (2005) Trends Biochem. Sci. 30, 605–610 [DOI] [PubMed] [Google Scholar]
- 26.Zaidi S. K., Young D. W., Montecino M. A., Lian J. B., van Wijnen A. J., Stein J. L., Stein G. S. (2010) Nat. Rev. Genet. 11, 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing H., Vanderford N. L., Sarge K. D. (2008) Nat. Cell Biol. 10, 1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Farrell P. H. (2001) Trends Cell Biol. 11, 512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan C. H., Russell P. (1993) EMBO J. 12, 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booher R. N., Holman P. S., Fattaey A. (1997) J. Biol. Chem. 272, 22300–22306 [DOI] [PubMed] [Google Scholar]
- 31.Fisher R. P., Morgan D. O. (1994) Cell 78, 713–724 [DOI] [PubMed] [Google Scholar]
- 32.Timofeev O., Cizmecioglu O., Settele F., Kempf T., Hoffmann I. (2010) J. Biol. Chem. 285, 16978–16990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blethrow J. D., Glavy J. S., Morgan D. O., Shokat K. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindqvist A., Rodríguez-Bravo V., Medema R. H. (2009) J. Cell Biol. 185, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J. M. (2003) EMBO J. 22, 6598–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandeis M., Rosewell I., Carrington M., Crompton T., Jacobs M. A., Kirk J., Gannon J., Hunt T. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4344–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodmer R., Barbel S., Sheperd S., Jack J. W., Jan L. Y., Jan Y. N. (1987) Cell 51, 293–307 [DOI] [PubMed] [Google Scholar]
- 38.Jack J., Dorsett D., Delotto Y., Liu S. (1991) Development 113, 735–747 [DOI] [PubMed] [Google Scholar]
- 39.Liu S., Jack J. (1992) Dev. Biol. 150, 133–143 [DOI] [PubMed] [Google Scholar]
- 40.Ludlow C., Choy R., Blochlinger K. (1996) Dev. Biol. 178, 149–159 [DOI] [PubMed] [Google Scholar]
- 41.van Wijnen A. J., Wright K. L., Lian J. B., Stein J. L., Stein G. S. (1989) J. Biol. Chem. 264, 15034–15042 [PubMed] [Google Scholar]
- 42.Holthuis J., Owen T. A., van Wijnen A. J., Wright K. L., Ramsey-Ewing A., Kennedy M. B., Carter R., Cosenza S. C., Soprano K. J., Lian J. B., Stein J. L., Stein G. S. (1990) Science 247, 1454–1457 [DOI] [PubMed] [Google Scholar]
- 43.van Wijnen A. J., Owen T. A., Holthuis J., Lian J. B., Stein J. L., Stein G. S. (1991) J. Cell. Physiol. 148, 174–189 [DOI] [PubMed] [Google Scholar]
- 44.Wright K. L., Dell'Orco R. T., van Wijnen A. J., Stein J. L., Stein G. S. (1992) Biochemistry 31, 2812–2818 [DOI] [PubMed] [Google Scholar]
- 45.van Wijnen A. J., van Gurp M. F., de Ridder M. C., Tufarelli C., Last T. J., Birnbaum M., Vaughan P. S., Giordano A., Krek W., Neufeld E. J., Stein J. L., Stein G. S. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11516–11521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon N. S., Bérubé G., Nepveu A. (2000) J. Biol. Chem. 275, 31325–31334 [DOI] [PubMed] [Google Scholar]
- 47.Moon N. S., Premdas P., Truscott M., Leduy L., Bérubé G., Nepveu A. (2001) Mol. Cell. Biol. 21, 6332–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coqueret O., Bérubé G., Nepveu A. (1998) EMBO J. 17, 4680–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goulet B., Baruch A., Moon N. S., Poirier M., Sansregret L. L., Erickson A., Bogyo M., Nepveu A. (2004) Mol. Cell 14, 207–219 [DOI] [PubMed] [Google Scholar]
- 50.Santaguida M., Ding Q., Bérubé G., Truscott M., Whyte P., Nepveu A. (2001) J. Biol. Chem. 276, 45780–45790 [DOI] [PubMed] [Google Scholar]
- 51.Santaguida M., Nepveu A. (2005) J. Biol. Chem. 280, 32712–32721 [DOI] [PubMed] [Google Scholar]
- 52.Sansregret L., Goulet B., Harada R., Wilson B., Leduy L., Bertoglio J., Nepveu A. (2006) Mol. Cell. Biol. 26, 2441–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K. A., Bindereif A., Green M. R. (1988) Gene Anal. Tech. 5, 22–31 [DOI] [PubMed] [Google Scholar]
- 54.Coqueret O., Bérubé G., Nepveu A. (1996) J. Biol. Chem. 271, 24862–24868 [DOI] [PubMed] [Google Scholar]
- 55.Coqueret O., Martin N., Bérubé G., Rabbat M., Litchfield D. W., Nepveu A. (1998) J. Biol. Chem. 273, 2561–2566 [DOI] [PubMed] [Google Scholar]
- 56.Michl P., Knobel B., Downward J. (2006) J. Biol. Chem. 281, 15138–15144 [DOI] [PubMed] [Google Scholar]
- 57.Truscott M., Denault J. B., Goulet B., Leduy L., Salvesen G. S., Nepveu A. (2007) J. Biol. Chem. 282, 30216–30226 [DOI] [PubMed] [Google Scholar]
- 58.Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. (1992) Cell 71, 875–886 [DOI] [PubMed] [Google Scholar]
- 60.Goulet B., Sansregret L., Leduy L., Bogyo M., Weber E., Chauhan S. S., Nepveu A. (2007) Mol. Cancer Res. 5, 899–907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.