Abstract
Although metabolic conditions associated with an increased AMP/ATP ratio are primary factors in the activation of 5′-adenosine monophosphate-activated protein kinase (AMPK), a number of recent studies have shown that increased intracellular levels of reactive oxygen species can stimulate AMPK activity, even without a decrease in cellular levels of ATP. We found that exposure of recombinant AMPKαβγ complex or HEK 293 cells to H2O2 was associated with increased kinase activity and also resulted in oxidative modification of AMPK, including S-glutathionylation of the AMPKα and AMPKβ subunits. In experiments using C-terminal truncation mutants of AMPKα (amino acids 1–312), we found that mutation of cysteine 299 to alanine diminished the ability of H2O2 to induce kinase activation, and mutation of cysteine 304 to alanine totally abrogated the enhancing effect of H2O2 on kinase activity. Similar to the results obtained with H2O2-treated HEK 293 cells, activation and S-glutathionylation of the AMPKα subunit were present in the lungs of acatalasemic mice or mice treated with the catalase inhibitor aminotriazole, conditions in which intracellular steady state levels of H2O2 are increased. These results demonstrate that physiologically relevant concentrations of H2O2 can activate AMPK through oxidative modification of the AMPKα subunit. The present findings also imply that AMPK activation, in addition to being a response to alterations in intracellular metabolic pathways, is directly influenced by cellular redox status.
Keywords: AMP-activated Kinase (AMPK), Inflammation, Lipopolysaccharide (LPS), Lung, Reactive Oxygen Species (ROS), Redox Signaling
Introduction
AMPK3 is a serine/threonine kinase that consists of three subunits, of which the α subunit has inducible kinase activity and the β and γ subunits have regulatory function. Formation of the αβγ complex is required for optimal allosteric activation of AMPK, which is induced by binding of AMP to the γ subunit (1–4). In addition to activation by AMP, phosphorylation of the Thr172 residue of the α subunit enhances kinase activity (5, 6). Recent studies have shown that the autoinhibitory domain (AID), located between amino acids 312 and 335 of the AMPKα subunit, is responsible for the lack of kinase activity under basal conditions (7–9), whereas AMP-induced conformational changes within the αβγ complex diminish function of the AID and lead to kinase activation.
The regulation of AMPK activity is primarily thought to result from alterations in the intracellular AMP/ATP ratio, arising from diminished ATP generation due to hypoxia, glucose deprivation, heat shock, or reduction in mitochondrial oxidative phosphorylation or from increased ATP consumption, such as occurs during strenuous exercise (2, 10–12). Once activated, AMPK can phosphorylate and modulate the function of essential metabolic pathways participating in the regulation of glucose and lipid homeostasis (13–15). A major effect of AMPK activation is in preserving energy for use under conditions where ATP is limiting (4, 16). AMPK activation appears to prevent or diminish inflammation-associated organ injury, including the development of atherosclerotic cardiovascular disease in diabetes (17), ischemia-induced cardiac dysfunction (18–20), and hepatic dysfunction in animal models of nonalcoholic steatohepatitis as well as in humans with this condition (21, 22). Our studies have also suggested that therapeutic approaches to increase AMPK activity diminish the severity of LPS-induced acute lung injury in mice (23, 24).
Although increased formation of reactive oxygen species (ROS) is generally thought to be associated with pathophysiological situations leading to cellular injury and organ dysfunction, recent studies have shown beneficial effects of ROS in modulating inflammation, including TLR4-induced neutrophil activation and LPS-associated acute lung injury (24–27). Several studies have demonstrated that increased intracellular concentrations of H2O2 result in activation of AMPK and enhancement of AMPK-mediated cellular adaptation (28–30), including maintenance of redox homeostasis (31, 32). In cardiac preconditioning studies, antioxidants diminished H2O2-associated activation of AMPK and resulted in increased severity of ischemia-reperfusion-induced cardiac heart injury (33).
Despite the ability of increased intracellular concentrations of H2O2 to induce AMPK activation in many cell types, the mechanism for this effect has not been well characterized. Whereas initial reports showed that H2O2-dependent activation of AMPK resulted from ATP depletion and increased AMP/ATP ratios (34), other studies demonstrated that increased intracellular concentrations of H2O2 were associated with activation of AMPK before or without alteration in the ATP/AMP ratio (35–37).
H2O2 can affect redox-sensitive signaling pathways as a result of oxidative modification of cysteine residues in proteins (38–40). We therefore hypothesized that a potential mechanism by which increased intracellular concentrations of H2O2 can activate AMPK is through oxidation of cysteines in one or more AMPK subunits. Our present experiments demonstrate that exposure to H2O2 is associated with cysteine oxidation in the AMPKα subunit and is able to directly activate AMPK.
EXPERIMENTAL PROCEDURES
Mice
Male C57BL/6, C3HeB/FeJ, or acatalasemic C3Ga.Cg-Cat B/J mice, 8–12 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept on a 12 h/12 h light/dark cycle with free access to food and water. All experiments were conducted in accordance with institutional review board-approved protocols (University of Alabama at Birmingham Institutional Animal Care and Use Committee).
Acute Lung Injury Model
Acute lung injury was induced by intratracheal administration of 1 mg/kg LPS in 50 μl of PBS as described previously (23–25, 41). Briefly, mice were anesthetized with isoflurane and then suspended by their upper incisors on a 60° incline board. The tongue was gently extended, and LPS solution was deposited into the pharynx (24, 42, 43). Mice were pretreated with saline or ATZ (500 mg/kg body weight dissolved in 0.9% saline) i.p., and 4 h later LPS (1 mg/kg) was administered intratracheally. Lungs were harvested 24 h after LPS administration.
Culture of Human Embryonic Kidney Cells
HEK 293 cells were maintained at 37 °C in 5% CO2 in RPMI 1640 growth medium (Invitrogen) that contained 8% fetal bovine serum (Atlanta Biologicals; Norcross, GA), l-glutamine (2 mm), penicillin (100 units/ml), and streptomycin (100 ng/ml) (Sigma). Prior to use in experiments, the cells were washed twice and incubated with RPMI 1640 medium (FBS, 0.5%) for 1 h and then treated as described in the figure legends.
Reagents and Antibodies
Purified human AMPKαβγ complex (>95% purity; specific activity, 737 nmol−1 min−1 mg−1) was obtained from SignalChem (Richmond, Canada). AMPKα, AMPKβ, or AMPKγ antibody was purchased from Cell Signaling (Beverly, MA). Biotinylated glutathione ethyl ester and streptavidin-agarose were purchased from Invitrogen, whereas hydrogen peroxide, glutathione, and aminotriazole (ATZ) were obtained from Sigma. Bio-Gel P10 was purchased from Bio-Rad. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside was purchased from Toronto Research Chemicals (Toronto, Canada).
Measurement of AMPK Activity
AMPK activity was determined using a radiometric assay and SAMS peptide substrate, as described previously (44) with minor modifications. Briefly, recombinant AMPKαβγ complex (25 ng/sample) or purified FLAG-AMPKα was incubated in kinase buffer (60 μl/sample) (MOPS (5 mm), β-glycerol-phosphatase (2.5 mm), MgCl2 (5 mm), 1 mm EGTA, and diethylene triamine pentaacetic acid (100 μm)) and SAMS peptide (5 μg/sample) for 10 min at room temperature. The phosphorylation of SAMS peptide was initiated by the inclusion of 0.2 μl of [32P]ATP and cold ATP (20 μm) mix, and samples were incubated at 30 °C. The reaction mix (2 μl) was transferred to phosphocellulose P81 at the times indicated in the figure legends. Air-dried phosphocellulose P81 was washed three times (10 min each wash) in phosphoric acid (1%) solution. The phosphocellulose P81 was then subjected to autoradiography, and dot density was determined using Alpha-Innotech software (Santa Clara, CA).
Western Blot Analysis of AMPK Subunits
Lung homogenates or extracts from HEK 293 cells were prepared in lysis buffer (Tris, pH 7.4 (50 mm), NaCl (150 mm), Nonidet P-40 (0.5% v/v), EDTA (1 mm), EGTA (1 mm), Na3VO4 (1 mm), NaF (50 mm), and protease inhibitors) and then sonicated and centrifuged at 10,000 × g for 15 min at 4 °C. The protein concentration in supernatants was determined using Bradford reagent (Bio-Rad) with BSA as a standard (24, 45). Samples were mixed with Laemmli sample buffer and boiled for 5 min. Equal amounts of protein were resolved by 8% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Immobilon P, Millipore, Billerica, MA). The membranes were probed with specific antibodies to AMPK subunits, followed by detection with horseradish peroxidase-conjugated anti-mouse or goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (SuperSignal; Pierce). Each experiment was carried out two or more times using HEK 293 cells or with lung homogenates obtained from separate groups of mice.
Imaging of DCF Fluorescence
Intracellular levels of ROS, including H2O2, were measured using the redox-sensitive probe DCFH-DA (46) in conjunction with fluorescent microscopy (24, 26, 38, 45, 47, 48). Briefly, HEK 293 cells (∼80% confluent) were incubated in a 4-well chambered coverglass (Nalge; Naperville, IL) with DCFH-DA (10 μm) for 60 min, followed by treatment with H2O2 (0 or 200 μm) or glucose oxidase (10 milliunits/ml) at 37 °C. Images were acquired at the indicated time periods (as described in the figure legends) by single bidirectional scans of live cells using a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics. The pinhole setting was 0.2 Airy units, and laser excitation was set for 5% to avoid dye photo-oxidation. The levels of fluorescence were averaged using SimplePCI software (Compix, Cranberry Township, PA). Images were processed using IPLab Spectrum and Adobe Photoshop (Adobe Systems) software.
S-Glutathionylation of AMPK
Purified AMPK (100 ng) in kinase buffer containing diethylene triamine pentaacetic acid (100 μm) was incubated with GSH-biotin (0 or 100 μm) for 15 min, followed by exposure to H2O2 (200 μm) for an additional 5 min. Samples were then boiled in Laemmli sample buffer (without DTT) for 5 min, resolved in non-reducing SDS-PAGE, followed by Western blot analysis with streptavidin-HRP. Membranes were subsequently reprobed with antibodies specific for AMPK subunits.
Detection of GSS-AMPK Adduct Formation in HEK 293 Cells
HEK 293 cells (2 × 106/ml) were incubated with ethyl ester GSH-biotin (6 mm) for 1.5 h. The cells were then washed twice with culture buffer to remove the excess of GSH and treated with H2O2 (0 or 300 μm) for 15 or 30 min. Cell lyses were prepared in the presence of N-ethylmaleimide (5 mm) and then passed through Bio-Gel P10 to remove free GSH-biotin and N-ethylmaleimide. The level of GSS-protein conjugates was determined using non-reducing Western blot analysis with streptavidin-HRP, whereas GSS-AMPK subunit levels were measured after pull-down with streptavidin-agarose (60 min at 4 °C), followed by reducing SDS-PAGE and Western blot analysis with antibodies to the AMPKα, AMPKβ, or AMPKγ subunits.
Labeling of AMPK Free Cysteine Thiols
The extent of free (unoxidized) cysteine residues within AMPK subunits was determined using the BIAM labeling assay (49–52). Briefly, cell extracts or lung extracts (0.4 mg/sample) obtained from control, acatalasemic, or ATZ-treated mice were incubated with BIAM (200 μm) for 30 min at room temperature, and then excess BIAM was removed by passing the extracts through Bio-Gel P10. Next, BIAM-protein conjugates were precipitated with streptavidin-agarose for 1 h at 4 °C. Samples were washed four times with lysis buffer containing 0.5% SDS to obtain specific pull-down of biotinylated proteins and to avoid potential contamination with unlabeled proteins. BIAM-protein adducts were extracted from streptavidin agarose by boiling in Laemmli sample buffer for 10 min and then subjected to reducing SDS-PAGE and Western blot analysis with antibodies to the AMPKα, AMPKβ, or AMPKγ subunits.
Metal-catalyzed Oxidation of Purified AMPK
Human purified AMPKαβγ complex (25 ng) was incubated with H2O2 (0 or 100 μm) in the presence or absence of Fe2+ (100 μm) or Cu1+ (100 μm) in kinase buffer (without diethylene triamine pentaacetic acid) for 10 min at 25 °C, followed by measurement of AMPK activity over the next 30 min.
Autophosphorylation of AMPK
Human purified recombinant AMPKαβγ complex (100 ng) was incubated in kinase buffer with H2O2 (0, 100, or 200 μm) for 10 min at room temperature, and then 0.2 μl of [32P]ATP and ATP (20 μm) were added to the cultures for an additional 30 min at 37 °C. Proteins were then subjected to SDS-PAGE and autoradiography.
Co-immunoprecipitation
Cells expressing FLAG-tagged AMPKα (WT) or AMPKα (amino acids 1–335) truncation were lysed in immunoprecipitation buffer (53) that preserves protein-protein interactions, followed by incubation of cell extracts with anti-FLAGM2 beads for 60 min at 4 °C. Beads were washed with immunoprecipitation buffer four times. The amount of AMPKβ subunit associated with AMPKα or AMPKα 1–335 was then determined by subsequent probing of the Western blot membrane with anti-FLAG and anti-AMPKβ antibodies.
Construction of Expression Plasmids and Recombinant Protein Expression
Full-length human AMPKα cDNA was purchased from Open Biosystems and cloned into 3XFLAG-CMV10 (Sigma) for mammalian expression. FLAG-tagged AMPKα and the C-terminal truncation mutants AMPKα 1–335 and AMPKα 1–312 were obtained by insertion of PCR products into 3XFLAG-CMV10. Mutation of cysteine 299, 304, or 312 to alanine within FLAG-tagged full-length AMPKα (WT) or truncated AMPKα (1–312 was performed using standard mutagenesis techniques. Transiently expressed FLAG-AMPKα, WT, or truncation mutants were purified using anti-M2 FLAG-agarose beads as described previously (54) and then subjected to Western blot analysis with anti-FLAGM2 or anti-phospho-Thr172-AMPK antibodies. In parallel experiments, beads containing FLAG-AMPKα (e.g. WT or truncation mutants) were washed twice with kinase buffer, and AMPK activity was determined using a radiometric assay and SAMS peptide as a substrate.
Measurement of Cellular Nucleotides
The levels of ATP, ADP, and AMP in HEK 293 cells were determined by etheno derivatization and subsequent HPLC analysis of the resulting fluorescent species, as described previously (55, 56).
Statistical Analyses
Experiments with purified AMPK or HEK 293 cells were each performed two or more times. Student's t test was used for comparisons between two groups, whereas Tukey's test was performed for comparisons between more than two groups, with p < 0.05 considered to be statistically significant. Mouse lung homogenates were obtained from two separate groups of control or acatalasemic mice or mice treated with ATZ (n = 3 mice in each group).
RESULTS
Exposure of HEK 293 Cells to H2O2 Results in Activation of AMPK
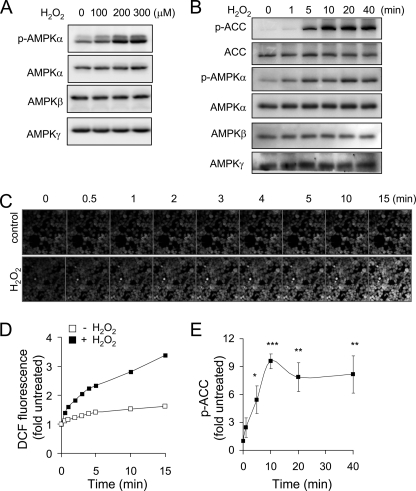
ROS, including H2O2, have been shown to activate AMPK in macrophages, neutrophils, and other cell populations. As shown in Fig. 1, A and B, exposure of HEK 293 cells to H2O2 resulted in activation of AMPK that was dose- and time-dependent (Fig. 1, A and B). Activation of AMPK occurred rapidly after cellular exposure to H2O2, with increased levels of phospho-Thr172-AMPK and phospho-Ser79-ACC being detected within ∼1–5 min after the addition of H2O2 to the cultures. The addition of H2O2 to HEK 293 cells resulted in enhanced DCF fluorescence, an indicator of increased intracellular ROS concentrations (Figs. 1, C and D), within 1 min, suggesting that the increase in the intracellular levels of H2O2 was responsible for the activation of AMPK in H2O2-exposed HEK 293 cells.
FIGURE 1.
Exposure to H2O2 induces rapid activation of AMPK and DCFH-DA oxidation in HEK 293 cells. A and B, representative Western blots show levels of the AMPKα, AMPKβ, and AMPKγ subunits, phospho-Thr172-AMPKα, ACC, and phospho-Ser79-ACC obtained from HEK 293 cells exposed to H2O2 (0, 100, 200, or 300 μm) for 60 min (B) or cells treated with H2O2 (250 μm) for 0–40 min (A). Confocal images (C) and mean DCF fluorescence (D) obtained from cells treated with H2O2 (250 μm) for 0–15 min. Confocal images of the same cell populations were acquired at the indicated time period. A second experiment provided similar results. E, quantitative analysis of phospho-Ser79-ACC (p-ACC) in cells treated as described in A. Shown is the mean ± S.D. (error bars), n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
H2O2 Induces S-Glutathionylation of AMPK Subunits
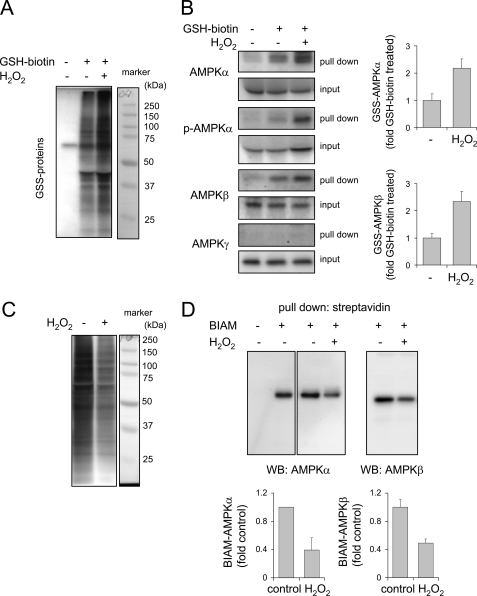
Alterations in intracellular concentrations of H2O2 can modulate the function of intracellular signaling pathways through oxidative modification, including S-glutathionylation, of cysteine residues (57, 58). To determine if exposure of HEK 293 cells to H2O2 had such effects on AMPK, cells loaded with ethyl ester GSH-biotin were incubated with H2O2. As shown in Fig. 2A, exposure to H2O2 resulted in an increase in the levels of GSS-protein adduct formation in HEK 293 cells.
FIGURE 2.
H2O2 induces oxidation of cysteine residues within AMPK subunits. A, HEK 293 cells loaded with or without EE-GSH-biotin were incubated with H2O2 (0 or 250 μm) for 20 min, and the amounts of GSS-protein adduct formation were determined using non-reducing SDS-PAGE and Western blot analysis with streptavidin-HRP. B, cell extracts obtained under the same conditions as in A were incubated with streptavidin-agarose, and the amounts of GSS-protein conjugates of the AMPKα, AMPKβ, and AMPKγ subunits as well as of phospho-Thr172-AMPKα (p-AMPKα) were determined by probing the Western blots with specific antibodies. Right, mean ± S.D. (error bars) obtained from two experiments. C, cellular proteins were incubated with or without H2O2, followed by incubation with BIAM. Western blot analysis with streptavidin-HRP was then performed. D, protein-BIAM adducts were purified using streptavidin pull-down and then subjected to Western blot analysis (WB) using anti-AMPKα or AMPKβ antibodies (mean ± S.D. obtained from two experiments).
We next determined whether H2O2 exposure produced S-glutathionylation of AMPK subunits. In these experiments, GSS-protein conjugates were precipitated using streptavidin-agarose, followed by Western blot analysis of AMPK subunits. As shown in Fig. 2B, there were increased amounts of GSS-phospho-Thr172-AMPKα and GSS-AMPKβ, but not of GSS-AMPKγ, in cells treated with H2O2. Similarly, increased oxidation of cysteine residues within the AMPKα and AMPKβ subunits was detected when cell extracts were incubated with BIAM and H2O2. Under these conditions, a decrease in BIAM-protein adduct formation, such as was found for the AMPKα and AMPKβ subunits after direct exposure to H2O2, indicated the presence of oxidized cysteine residues that were unable to react with BIAM (Fig. 2, C and D). These results show that activation of AMPK by H2O2 is associated with enhanced oxidative modification of both the AMPKα and AMPKβ subunits.
Activation of AMPK as Well as Cysteine Oxidation in the AMPKα Subunit Precede Decline in ATP levels in H2O2-exposed HEK 293 Cells
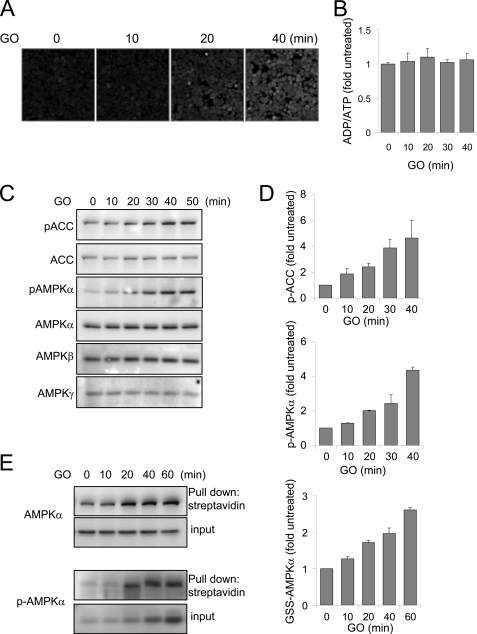
Although the above experiments demonstrated that cellular exposure to H2O2 resulted in activation as well as oxidative modification of AMPK, those studies do not show that oxidative modification precedes activation of AMPK or is responsible for kinase activation. An alternate explanation is that activation of AMPK could have been produced by decreases in cellular levels of ATP and alteration in the ATP/AMP ratio as a result of incubation of HEK 293 cells with H2O2, with oxidative modification of AMPK occurring as a subsequent and independent event. Indeed, we found that exposure of HEK 293 cells to H2O2 (250 μm) for 30 min resulted in about a 30–40% decrease in intracellular levels of ATP. Because of the rapidity with which AMPK became activated after incubation of HEK 293 cells with H2O2, we measured AMPK activation and cellular levels of ATP in HEK 293 cells exposed to glucose oxidase (GO)-generated H2O2, a methodology that, in contrast to direct cellular exposure to H2O2, results in a more gradual increase in H2O2 levels in the cell culture media as well as sustained elevations in intracellular steady state concentrations of H2O2 (26, 59). As shown in Fig. 3, inclusion of GO (10 milliunits/ml) in the cell cultures time-dependently induced DCF fluorescence and resulted in increased phosphorylation of AMPKα and ACC, as well as S-glutathionylation of both AMPKα and phospho-Thr172-AMPKα. Importantly, activation and oxidation of the AMPKα subunit was present before any changes in intracellular concentrations of ADP and ATP or of the ATP/ADP ratio occurred (Fig. 3B). These results show that the stimulatory effects of H2O2 on AMPK activity are not associated with diminished cellular ATP concentrations.
FIGURE 3.
H2O2 induces oxidation and activation of AMPK without depletion of cellular ATP. A–D, HEK 293 cells loaded with DCFH-DA were cultured with GO (10 milliunits/ml) for 0, 10, 20, or 40 min, and then images were acquired using confocal microscopy (A). B shows the ADP/ATP ratios in cells incubated with GO (10 milliunits/ml) for the indicated time periods, whereas C and D show representative Western blots of AMPK subunits and phospho-ACC (pACC) and mean ± S.D. (error bars) obtained from two experiments that utilized HEK 293 cells treated with GO for 0–50 min. E, HEK 293 cells were loaded with EE-GSH-biotin and then GO (10 milliunits/ml) included in culture medium for the indicated time period. Cell extracts were subjected to pull-down with streptavidin-agarose, followed by Western blotting with antibodies specific for AMPKα or phospho-Thr172-AMPK (p-AMPK) (input, levels of AMPK or phospho-Thr172 in cell extract prior to pull-down assay; Pull down, the amount of AMPK or phospho-Thr172-AMPK obtained after precipitation with streptavidin-agarose). Shown is the mean ± S.D. obtained from two experiments.
Assembly of the AMPKαβγ complex can be potentially affected by exposure to increased concentrations of H2O2. However, incubation of cells with H2O2 did not appear to modify the composition of AMPKαβγ complexes (supplemental Fig. S1A).
Direct Exposure of AMPK to H2O2 Increases Kinase Activity
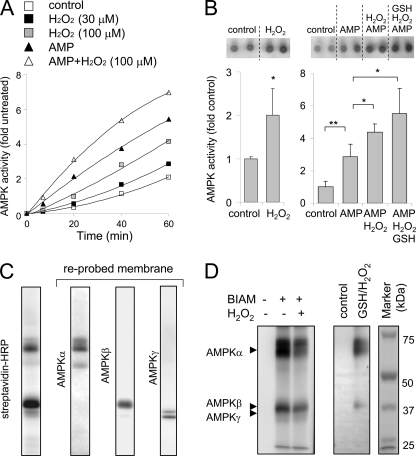
In order to determine if H2O2 could directly activate AMPK, we incubated recombinant AMPKαβγ complex with H2O2 and then determined kinase activity. As shown in Fig. 4, A and B, and supplemental Fig. S2, A and B, exposure of the AMPKαβγ complex to H2O2 dose-dependently increased AMPK activity, even in the presence of AMP. In additional experiments, we have found that H2O2-dependent activation of AMPK resulted in autophosphorylation of the AMPKα and AMPKβ subunits (supplemental Fig. S2C). These results are consistent with previous studies showing that activated AMPK undergoes autophosphorylation, with the α and β subunits being affected (60).
FIGURE 4.
H2O2 directly activates AMPK. A and B, recombinant AMPKαβγ complex was incubated with H2O2 (0, 30, or 100 μm) for 10 min, followed by inclusion of AMP (0 or 200 μm) for an additional 10 min. A shows the time-dependent increase in phosphorylation of SAMS peptide by AMPK in the presence or absence of H2O2 or AMP and H2O2, whereas B shows representative autoradiograms (upper panels) and quantitative analysis of SAMS phosphorylation by AMPK (bottom panel). Shown is the mean ± S.D. (error bars), n = 3; *, p < 0.05; **, p < 0.01). AMPK was incubated with or without H2O2 and a combination of H2O2, AMP, or GSH as indicated. GSH (0 or 100 μm) was added to the cultures for 15 min after H2O2 or H2O2 and AMP exposure. C and D, in C, recombinant AMPKαβγ complex (0.3 μg) was incubated with BIAM (10 μm) for 10 min, followed by Western blotting with streptavidin-HRP. The membrane was then reprobed with antibodies specific for the AMPKα, AMPKβ, and AMPKγ subunits. D, AMPKαβγ complex was incubated with H2O2 (0 or 100 μm) for 10 min, followed by inclusion of BIAM (0 or 10 μm) in the cultures for an additional 10 min. AMPKαβγ complex was also incubated with H2O2 (0 or 100 μm) for 10 min, followed by GSH-biotin (0 or 200 μm) for 10 min. Proteins were resolved using non-reducing SDS-PAGE, and the amounts of AMPK-BIAM or GSS-AMPK adduct formation were determined using Western blot analysis with streptavidin-HRP.
Consistent with results obtained from experiments that utilized cells cultured with H2O2 (Fig. 2), the increase in kinase activity produced by direct incubation of the AMPKαβγ complex with H2O2 was associated with oxidative modification of the α and β subunits, as shown by a decrease in BIAM adduct formation as well as increased S-glutathionylation of the AMPKα and AMPKβ subunits (Fig. 4, C and D). These results indicate that H2O2 can directly increase AMPK activity and that such activation is accompanied by oxidative modification of cysteine residues within the AMPKα and AMPKβ subunits.
Hydrogen Peroxide Can Activate AMPKα without Participation of β or γ Subunits
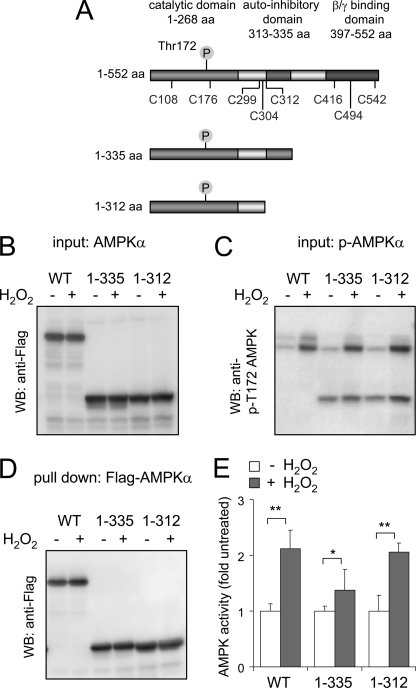
To determine whether H2O2-induced oxidation of the AMPKα subunit induces kinase activation independently of the effects of H2O2 on the other AMPK subunits, we transiently expressed AMPKα WT as well as AMPK with truncation of the C-terminal region (AMPKα 1–335 or AMPKα 1–312) in HEK 293 cells (Fig. 5). Deletion of the C-terminal region of the AMPKα subunit diminished complex formation between the α and β subunits (supplemental Fig. S1B), consistent with previous studies that have shown the importance of the β/γ binding domain located within the amino acids 397–552 region of AMPKα (7, 61). Exposure of HEK 293 cells to H2O2 resulted in increased activity and Thr172 phosphorylation of both wild type and truncated 1–335 or 1–312 AMPKα (Fig. 5E).
FIGURE 5.
A, human AMPK. The AMPKα subunit with the location of cysteines, phosphorylation site Thr172, catalytic domain, AID, and βγ binding domain and C-terminal truncation sites of AMPKα1 as used in the experiments is shown. B–D, Western blots (WB) of transiently expressed FLAG-AMPKα WT, AMPK 1–335, or AMPK 1–312 obtained from HEK 293 cells treated with H2O2 (0 or 250 μm) for 15 min. B and C show the level of total AMPK by probing the membrane with anti-FLAG antibodies or with antibodies to phospho-Thr172, whereas D shows the amount of FLAG-AMPKα or AMPKα truncation mutants obtained after pull-down with anti-FLAG-agarose. E, FLAG-tagged AMPKα (WT or truncation mutants) was subjected to pull-down, and then AMPK activity was determined using a radiometric assay with SAMS peptide as a substrate. Shown is the mean ± S.D. (error bars), n = 3. *, p < 0.05; **, p < 0.01 compared with untreated. aa, amino acids.
H2O2-dependent Oxidative Modification of Cysteine Thiols Results in Activation of AMPKα
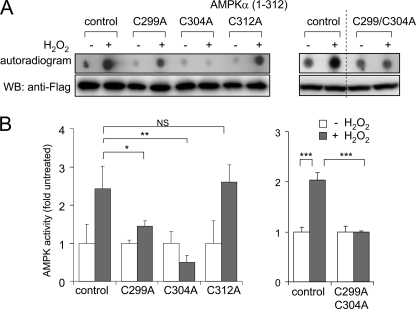
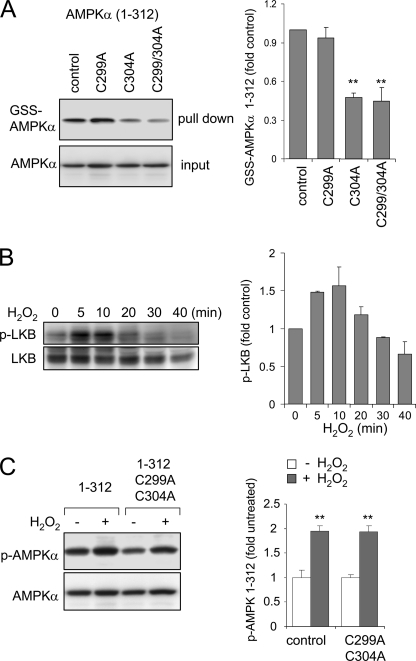
As shown in Fig. 5, exposure of the AMPKα subunit to H2O2 directly increases kinase activity even after elimination of the β/γ binding domain or of the AID. In particular, incubation with H2O2 still resulted in activation of truncated AMPKα 1–335 or AMPKα 1–312. There are three cysteine residues at the C-terminal end of the AMPKα 1–312 mutant that may be oxidatively modified by exposure to H2O2 and contribute to the kinase activation by H2O2 (i.e. Cys299, Cys304, and Cys312). To examine a potential role for these cysteines in H2O2-dependent regulation of AMPKα activity, point mutations of each cysteine to alanine were performed. When compared with the activation of AMPK 1–312 by exposure to H2O2, there was a marked decrease in the effects of H2O2 on kinase activity when Cys299 was mutated to an alanine. Mutation of Cys304 completely prevented activation of AMPKα 1–312 by H2O2. However, mutation of Cys312 did not result in any alteration in H2O2-induced kinase activation (Fig. 6). Mutation of both Cys299 and Cys304 blocked H2O2-induced activation of AMPKα (Fig. 6). Mutation of Cys299 had no effect on GSS-AMPK 1–312 adduct formation, suggesting that Cys304 was a specific target of H2O2-induced S-glutathionylation. Next, we determined if mutation of Cys299 and Cys312 affected the phosphorylation of Thr172 in AMPKα. As shown in Fig. 7C, exposure of cells to H2O2 produced similar levels of phosphorylation of AMPK 1–312 as compared with mutant AMPK 1–312 Cys299/304. This result suggests that oxidative modification of AMPKα, subsequent to phosphorylation, is an essential step in kinase activation.
FIGURE 6.
Effects of H2O2 on AMPK kinase activity in the AMPKα 1–312 truncation mutant and AMPKα 1–312(C299A), 1–312(C304A), 1–312(C312A), or 1–312(C299A/C304A) mutants. A and B, FLAG-tagged AMPKα WT or mutants were transiently expressed in HEK 293 cells, and the cells were treated with H2O2 (0 or 250 μm) for 15 min. Cell extracts were analyzed with Western blots (WB) using anti-FLAG antibodies. Kinase activity was determined using a radiometric assay with SAMS peptide as the substrate. A, representative autoradiograms (top) and Western blots of FLAG-AMPK (bottom). Quantitative analysis of kinase activity is shown in B. Shown is the mean ± S.D. (error bars), n = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, not significant.
FIGURE 7.
Effects of H2O2 on S-glutathionylation and phosphorylation of AMPKα 1–312 and AMPKα 1–312 with mutated C299A and C304A. A, a representative Western blot shows the amounts of GSS-AMPKα 1–312 and GSS-AMPKα 1–312 with mutated C299A and C304A obtained from HEK 293 cells loaded with biotin-GSH-EE and then treated with H2O2 (0 or 250 μm) for 15 min (mean ± S.D. (error bars), n = 3; **, p < 0.01). B, LKB1 phosphorylation (p-LKB) and total LKB1 levels were determined in HEK 293 cells treated with H2O2 (250 μm) for the indicated time. Representative Western blots are shown (mean ± S.D. obtained from two experiments). C, a representative Western blot of total and phosphorylated AMPKα 1–312 (p-AMPKα) and AMPKα 1–312 with mutated C299A and C304A obtained from HEK 293 cells treated with H2O2 for 15 min (mean ± S.D., n = 3; **, p < 0.01).
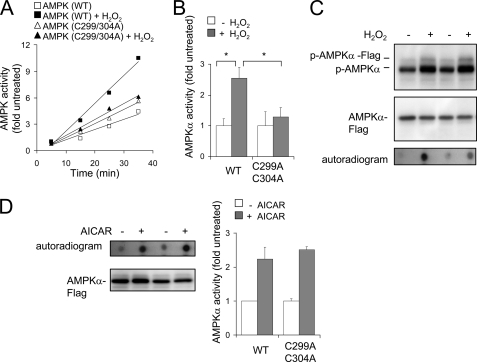
In additional experiments, we found that mutation of Cys299 and Cys304 in full-length AMPKα (amino acids 1–552) also diminished the ability of H2O2 but not 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, to induce kinase activity (Fig. 8).
FIGURE 8.
Effects of H2O2 on the kinase activity of wild type and mutant C299A/C304A AMPKα. A–C, AMPKα-FLAG WT or mutant AMPKα-FLAG C299A/C304A was transiently expressed in HEK 293 cells, and then the cells were treated with GO (0 or 10 milliunits/ml) for 30 min. A and B show the rate of SAMS phosphorylation by WT or mutant AMPK and mean ± S.D. (error bars) of AMPK kinase activity (n = 3; *, p < 0.05). C, Western blots of phospho-Thr172-AMPKα (p-AMPKα) and total AMPKα obtained from control cells and cells cultured with GO (10 milliunits/ml) for 30 min. D, a representative Western blot of AMPKα-FLAG activation and mean ± S.D. of AMPK kinase activity obtained from cells transiently expressing WT or mutant (C299A/C304A) AMPKα and cultured with or without 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) (0 or 1 mm) for 90 min.
AMPK Activity Is Increased in the Lungs of Acatalasemic Mice or Mice with Pharmacologically Induced Inhibition of Catalase
Previous studies have found that H2O2 at physiologically relevant concentrations can produce activation of AMPK in vivo (33). Recently, we have shown that treatment of neutrophils with ATZ, an inhibitor of catalase, resulted in increased intracellular steady state levels of H2O2 as well as diminished LPS-induced proinflammatory responses, including decreased nuclear translocation of NF-κB and expression of proinflammatory cytokines (25). Given the above studies showing that direct exposure of AMPK to H2O2 as well as incubation of HEK 293 cells with H2O2 resulted in oxidation of the AMPKα subunit and enhanced kinase activity, we hypothesized that in vivo conditions that produce increased intracellular concentrations of H2O2 would also be associated with oxidative modifications of the AMPKα subunit and increased kinase activity.
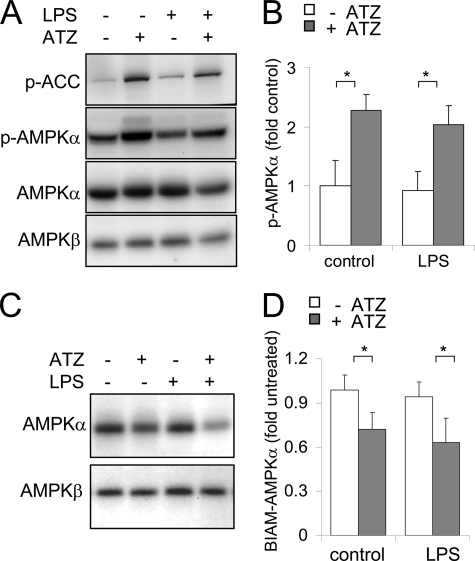
As shown in Fig. 9, administration of ATZ to mice resulted in increased levels in the lungs of phosphorylated Thr172-AMPK and AMPK activation, as shown by increased levels of the phosphorylated form of ACC (phospho-Ser79-ACC), a downstream target of AMPK. Measurement of free cysteine thiols with BIAM labeling showed decreased amounts of BIAM-AMPKα and BIAM-AMPKβ adduct formation in lung homogenates of mice given ATZ as compared with control mice treated with saline. Intratracheal LPS administration had little or no effect on AMPK phosphorylation in the lungs, whereas administration of ATZ before injection of LPS resulted in increased kinase activity. In a similar manner to acute inhibition of catalase by ATZ, increased phosphorylation and oxidation of AMPK were present in lung homogenates from acatalasemic mice (supplemental Fig. S4) compared with control mice with normal catalase function.
FIGURE 9.
Mice with pharmacologic inhibition of catalase activity or acatalasemic mice have increased activation and oxidation of AMPKα subunit in the lungs. A and B, mice were given 50 μl of saline or LPS (1 mg/kg) in 50 μl of saline intratracheally and were treated with ATZ i.p. at 500 mg/kg. ATZ was administered 4 h before intratracheal saline or LPS administration. Lungs were harvested 24 h after treatment of the mice with LPS, and the levels of the AMPKα or AMPKβ subunits, phospho-Thr172-AMPKα, ACC, and phospho-Ser79-ACC (p-ACC), were determined using Western blot analysis. Representative Western blots are shown in A, whereas quantitative analysis of AMPK phosphorylation in lung homogenates is shown in B (mean ± S.D. values were obtained from 3 mice/group; *, p < 0.05). C, lung homogenates obtained from control saline-treated mice or mice given LPS, ATZ, or ATZ and LPS were incubated with BIAM, and levels of AMPK-BIAM adduct formation were determined by Western blot analysis with streptavidin-HRP. The mean ± S.D. is shown using results from two experiments.
DISCUSSION
In this study, we demonstrated that H2O2 can directly activate AMPK in vitro and in vivo through a mechanism associated with enhanced oxidative modification, including S-glutathionylation of cysteine residues, of the AMPKα subunit. Previous reports had shown that exposure of various cell populations to H2O2 or to agents that produce increased intracellular concentrations of H2O2, such as by inhibiting mitochondrial electron transport, result in increased AMPK activity (29, 35, 37, 62). The ability of H2O2 to activate AMPK has been hypothesized to be indirect and to occur through decreasing intracellular levels of ATP and increasing the ratio of AMP to ATP, thereby enhancing binding of AMP to the AMPKγ subunit with resultant allosteric activation of the AMPKα kinase domain (34). A recent study that utilized an AMP-insensitive mutant of the AMPKγ subunit suggested that the stimulatory effects of H2O2 on AMPK activation are mediated by a diminished ATP/ADP ratio (63). However, in the present experiments, we found that exposure of cells to increased levels of H2O2 activated and oxidized AMPK before any decrease in ATP levels or in the ATP/ADP ratio occurred. Moreover, we confirmed that H2O2 could directly activate AMPK by demonstrating that incubation of the AMPKαβγ complex with H2O2 increased kinase activity. However, because exposure of HEK 293 and other cell populations to H2O2 also results in diminished intracellular ATP levels and increased AMP/ATP ratios, in addition to increasing intracellular concentrations of H2O2, the ability of H2O2 to directly activate AMPK does not necessarily imply that this is the major mechanism by which increased generation of H2O2 produces activation of AMPK in vivo. Future experiments will be necessary to delineate the relative importance of direct oxidation of the AMPKα subunit as compared with alterations in AMP/ATP ratios in activating AMPK during pathophysiologic conditions, such as ischemia-reperfusion injury, that are associated with increased production of H2O2.
Although exposure to H2O2 resulted in oxidative modification and S-glutathionylation of both the α and β subunits of AMPK, H2O2-induced modification of the AMPKα subunit alone was sufficient to increase kinase activity. Of note, exposure of truncated AMPKα (amino acids 1–335), which lacks the binding domain for interaction with the AMPKβ and AMPKγ subunits, to H2O2 still increased AMPK activity, indicating that association with the β and γ subunits was not necessary for AMPK activation by H2O2. Recent studies have shown that interaction between of the AID and α-helix C region of the AMPKα subunit is responsible for retention of the inactive “open” conformation of the AMPKα subunit within the AMPKαβγ complex (7–9). However, exposure of AMPKα or AMPKα truncation mutants lacking the AID to H2O2 resulted in enhanced AMPK kinase activity, showing that the AID is unlikely to play an important role in this effect. Mutation of cysteine 299 decreased and mutation of cysteine 304 totally blocked the activation of AMPKα by H2O2, suggesting that oxidative modification of these two cysteines plays an important role in the ability of H2O2 to induce activation of AMPK.
Although our results show that direct exposure to H2O2 enhances the kinase activity of AMPK, enhanced phosphorylation of AMPK was also present under such conditions. In particular, incubation of the AMPKαβγ complex with H2O2 dose-dependently increased phosphorylation of the AMPKα and AMPKβ subunits. Such findings are consistent with previous studies that showed that activated AMPK undergoes autophosphorylation during activation (60).
Although H2O2 is a relatively weak oxidant, extracellularly generated H2O2 is capable of rapidly crossing cellular membranes to oxidize redox-sensitive cysteines of intracellular proteins and to modulate their activity in signaling pathways (38). The results of the present experiments, and particularly of those showing that direct exposure of the AMPKαβγ complex or of the AMPKα subunit to H2O2 increased kinase activity and diminished BIAM adduct formation, suggest that the mechanism by which H2O2 induces such effects is through oxidative modification of vulnerable cysteine residues. The ability of H2O2 to produce S-glutathionylation of the AMPKα and AMPKβ subunits is consistent with this hypothesis. Although the present findings suggest that exposure to H2O2 alone is sufficient to oxidatively modify and activate AMPK, it is possible that other ROS, such as hydroxyl radical, derived from H2O2 contribute to these effects. However, we found that metal-dependent generation of OH• in vitro diminished the activity of AMPK (supplemental Fig. S3). Such results suggest that H2O2 itself, rather than derived strong oxidants, is responsible for activation of AMPK under pathophysiologic in vivo conditions associated with increased generation of ROS.
Similar to the effects of H2O2 in cell cultures, we found that increased intracellular concentrations of H2O2 in the lungs under in vivo conditions also were associated with AMPK activation (64). A role for H2O2 in modulating AMPK activity in vivo was previously reported after cardiac ischemia, when increased levels of H2O2 in the heart were accompanied by activation of AMPK and protection from a second ischemic event (1, 65–68). Consistent with our experiments with purified AMPK and with HEK 293 cells, we found activation of AMPK and oxidative modification of the AMPKα subunit in the lungs of acatalasemic mice and in mice treated with the catalase inhibitor ATZ, conditions in which intracellular concentrations of H2O2 are elevated (25).
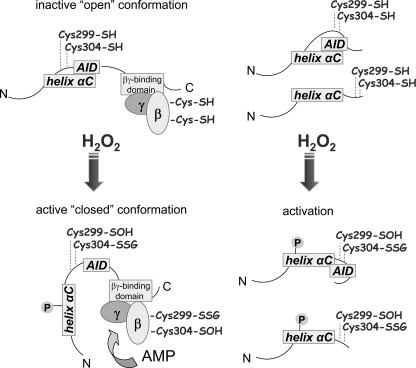
Our experiments demonstrate a novel mechanism for AMPK activation that involves oxidative modification of the AMPKα subunit as a result of direct exposure to H2O2 (Fig. 10). Previous studies have shown that activation of AMPK as well as increased intracellular concentrations of H2O2 have potent anti-inflammatory properties, including diminished severity of LPS-induced acute lung injury (25). The present experiments suggest that at least one mechanism by which H2O2 exerts its anti-inflammatory effects in vivo is through directly activating AMPK. Future studies will be necessary to determine if the primary mechanism for the anti-inflammatory effects of increased intracellular concentrations of H2O2 is through activation of AMPK.
FIGURE 10.
Putative mechanism of AMPK activation by H2O2. The AMPKαβγ complex is in an “open” inactive state, whereas H2O2 induces allosteric rearrangement to the active “closed” conformation as a result of H2O2-dependent oxidative modification of cysteine residues (-SOH), including S-glutathionylation (-SSG). Such oxidative modification, followed by dissociation of AID from α-helix C and activation of AMPKα, can be achieved without binding of β/γ subunit. In the heterotrimeric AMPK complex, oxidative modification of the α and β subunits can also facilitate AMP-dependent activation of the kinase domain.
Supplementary Material
Acknowledgment
We thank Dr. Jack Lancaster, Jr. for helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL62221, HL76206, and GM87748 (to E. A.) and P01-HL034322 (to E. R. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- AMPK
- 5′-adenosine monophosphate-activated protein kinase
- ROS
- reactive oxygen species
- ATZ
- 3-amino-1,2,4-triazole
- AID
- autoinhibitory domain
- GO
- glucose oxidase
- DCF
- 2′,7′-dichlorodihydrofluorescein
- DCF-DA
- 2′,7′-dichlorodihydrofluorescein diacetate
- GSS
- S-glutathionylation of
- BIAM
- biotinylated iodoacetamide
- ACC
- acetyl-CoA carboxylase.
REFERENCES
- 1.Baron S. J., Li J., Russell R. R., 3rd, Neumann D., Miller E. J., Tuerk R., Wallimann T., Hurley R. L., Witters L. A., Young L. H. (2005) Circ. Res. 96, 337–345 [DOI] [PubMed] [Google Scholar]
- 2.Hardie D. G., Hawley S. A., Scott J. W. (2006) J. Physiol. 574, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. (2004) J. Clin. Invest. 113, 274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towler M. C., Hardie D. G. (2007) Circ. Res. 100, 328–341 [DOI] [PubMed] [Google Scholar]
- 5.Stein S. C., Woods A., Jones N. A., Davison M. D., Carling D. (2000) Biochem. J. 345, 437–443 [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. (1996) J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 7.Crute B. E., Seefeld K., Gamble J., Kemp B. E., Witters L. A. (1998) J. Biol. Chem. 273, 35347–35354 [DOI] [PubMed] [Google Scholar]
- 8.Pang T., Xiong B., Li J. Y., Qiu B. Y., Jin G. Z., Shen J. K., Li J. (2007) J. Biol. Chem. 282, 495–506 [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Jiao Z. H., Zheng L. S., Zhang Y. Y., Xie S. T., Wang Z. X., Wu J. W. (2009) Nature 459, 1146–1149 [DOI] [PubMed] [Google Scholar]
- 10.Evans A. M., Mustard K. J., Wyatt C. N., Peers C., Dipp M., Kumar P., Kinnear N. P., Hardie D. G. (2005) J. Biol. Chem. 280, 41504–41511 [DOI] [PubMed] [Google Scholar]
- 11.Yun H., Lee M., Kim S. S., Ha J. (2005) J. Biol. Chem. 280, 9963–9972 [DOI] [PubMed] [Google Scholar]
- 12.Winder W. W., Holmes B. F., Rubink D. S., Jensen E. B., Chen M., Holloszy J. O. (2000) J. Appl. Physiol. 88, 2219–2226 [DOI] [PubMed] [Google Scholar]
- 13.Dolinsky V. W., Dyck J. R. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H2557–H2569 [DOI] [PubMed] [Google Scholar]
- 14.Long Y. C., Zierath J. R. (2006) J. Clin. Invest. 116, 1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie D. G., Sakamoto K. (2006) Physiology 21, 48–60 [DOI] [PubMed] [Google Scholar]
- 16.Hardie D. G., Scott J. W., Pan D. A., Hudson E. R. (2003) FEBS Lett. 546, 113–120 [DOI] [PubMed] [Google Scholar]
- 17.Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M. (2002) N. Engl. J. Med. 346, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. (2005) Nat. Med. 11, 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin E. J., Schram K., Zheng X. L., Sweeney G. (2009) J. Cell. Physiol. 221, 490–497 [DOI] [PubMed] [Google Scholar]
- 20.Gundewar S., Calvert J. W., Jha S., Toedt-Pingel I., Ji S. Y., Nunez D., Ramachandran A., Anaya-Cisneros M., Tian R., Lefer D. J. (2009) Circ. Res. 104, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H. Z., Yang S. Q., Chuckaree C., Kuhajda F., Ronnet G., Diehl A. M. (2000) Nat. Med. 6, 998–1003 [DOI] [PubMed] [Google Scholar]
- 22.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Zoli M., Melchionda N. (2001) Lancet 358, 893–894 [DOI] [PubMed] [Google Scholar]
- 23.Zhao X., Zmijewski J. W., Lorne E., Liu G., Park Y. J., Tsuruta Y., Abraham E. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L497–L504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Siegal G. P., Abraham E. (2008) Am. J. Respir. Crit. Care Med. 178, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Abraham E. (2009) Am. J. Respir. Crit. Care Med. 179, 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zmijewski J. W., Zhao X., Xu Z., Abraham E. (2007) Am. J. Physiol. Cell Physiol. 293, C255–C266 [DOI] [PubMed] [Google Scholar]
- 27.Strassheim D., Asehnoune K., Park J. S., Kim J. Y., He Q., Richter D., Mitra S., Arcaroli J., Kuhn K., Abraham E. (2004) Am. J. Physiol. Cell Physiol. 286, C683–C692 [DOI] [PubMed] [Google Scholar]
- 28.Horie T., Ono K., Nagao K., Nishi H., Kinoshita M., Kawamura T., Wada H., Shimatsu A., Kita T., Hasegawa K. (2008) J. Cell. Physiol. 215, 733–742 [DOI] [PubMed] [Google Scholar]
- 29.Irrcher I., Ljubicic V., Hood D. A. (2009) Am. J. Physiol. Cell Physiol. 296, C116–C123 [DOI] [PubMed] [Google Scholar]
- 30.Sandström M. E., Zhang S. J., Bruton J., Silva J. P., Reid M. B., Westerblad H., Katz A. (2006) J. Physiol. 575, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz E., Dopheide J., Schuhmacher S., Thomas S. R., Chen K., Daiber A., Wenzel P., Münzel T., Keaney J. F., Jr. (2008) Circulation 118, 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo S. L., Moncada S. (2009) Biochem. J. 421, 163–169 [DOI] [PubMed] [Google Scholar]
- 33.Lamberts R. R., Onderwater G., Hamdani N., Vreden M. J., Steenhuisen J., Eringa E. C., Loer S. A., Stienen G. J., Bouwman R. A. (2009) Circulation 120, S10–S15 [DOI] [PubMed] [Google Scholar]
- 34.Choi S. L., Kim S. J., Lee K. T., Kim J., Mu J., Birnbaum M. J., Soo Kim S., Ha J. (2001) Biochem. Biophys. Res. Commun. 287, 92–97 [DOI] [PubMed] [Google Scholar]
- 35.Emerling B. M., Weinberg F., Snyder C., Burgess Z., Mutlu G. M., Viollet B., Budinger G. R., Chandel N. S. (2009) Free Radic. Biol. Med. 46, 1386–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyoda T., Hayashi T., Miyamoto L., Yonemitsu S., Nakano M., Tanaka S., Ebihara K., Masuzaki H., Hosoda K., Inoue G., Otaka A., Sato K., Fushiki T., Nakao K. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E166–E173 [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Dong Y., Xu J., Xie Z., Wu Y., Song P., Guzman M., Wu J., Zou M. H. (2008) Circ. Res. 102, 328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dröge W. (2002) Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 39.Giustarini D., Rossi R., Milzani A., Colombo R., Dalle-Donne I. (2004) J. Cell Mol. Med. 8, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. (2007) Free Radic. Biol. Med. 43, 883–898 [DOI] [PubMed] [Google Scholar]
- 41.Foster W. M., Walters D. M., Longphre M., Macri K., Miller L. M. (2001) J. Appl. Physiol. 90, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 42.Tsuruta Y., Park Y. J., Siegal G. P., Liu G., Abraham E. (2007) J. Immunol. 179, 7079–7086 [DOI] [PubMed] [Google Scholar]
- 43.Brass D. M., Hollingsworth J. W., McElvania-Tekippe E., Garantziotis S., Hossain I., Schwartz D. A. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 293, L77–L83 [DOI] [PubMed] [Google Scholar]
- 44.Davies S. P., Carling D., Hardie D. G. (1989) Eur. J. Biochem. 186, 123–128 [DOI] [PubMed] [Google Scholar]
- 45.Zmijewski J. W., Lorne E., Banerjee S., Abraham E. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L624–L634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrona M., Patel K., Wardman P. (2005) Free Radic. Biol. Med. 38, 262–270 [DOI] [PubMed] [Google Scholar]
- 47.Watanabe N., Zmijewski J. W., Takabe W., Umezu-Goto M., Le Goffe C., Sekine A., Landar A., Watanabe A., Aoki J., Arai H., Kodama T., Murphy M. P., Kalyanaraman R., Darley-Usmar V. M., Noguchi N. (2006) Am. J. Pathol. 168, 1737–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zmijewski J. W., Moellering D. R., Le Goffe C., Landar A., Ramachandran A., Darley-Usmar V. M. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H852–H861 [DOI] [PubMed] [Google Scholar]
- 49.Kim J. R., Yoon H. W., Kwon K. S., Lee S. R., Rhee S. G. (2000) Anal. Biochem. 283, 214–221 [DOI] [PubMed] [Google Scholar]
- 50.Choi K. S., Park S. Y., Baek S. H., Dey-Rao R., Park Y. M., Zhang H., Ip C., Park E. M., Kim Y. H., Park J. H. (2006) Prep Biochem. Biotechnol. 36, 65–79 [DOI] [PubMed] [Google Scholar]
- 51.Cross J. V., Templeton D. J. (2006) Antioxid. Redox Signal. 8, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 52.Ying J., Clavreul N., Sethuraman M., Adachi T., Cohen R. A. (2007) Free Radic. Biol. Med. 43, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee S., Zmijewski J. W., Lorne E., Liu G., Sha Y., Abraham E. (2010) J. Biol. Chem. 285, 2665–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zmijewski J. W., Banerjee S., Abraham E. (2009) J. Biol. Chem. 284, 22213–22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreda S. M., Okada S. F., van Heusden C. A., O'Neal W., Gabriel S., Abdullah L., Davis C. W., Boucher R. C., Lazarowski E. R. (2007) J. Physiol. 584, 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mieyal J. J., Gallogly M. M., Qanungo S., Sabens E. A., Shelton M. D. (2008) Antioxid. Redox Signal. 10, 1941–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shelton M. D., Chock P. B., Mieyal J. J. (2005) Antioxid. Redox Signal. 7, 348–366 [DOI] [PubMed] [Google Scholar]
- 59.Mueller S., Weber A., Fritz R., Mütze S., Rost D., Walczak H., Völkl A., Stremmel W. (2002) Biochem. J. 363, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchelhill K. I., Michell B. J., House C. M., Stapleton D., Dyck J., Gamble J., Ullrich C., Witters L. A., Kemp B. E. (1997) J. Biol. Chem. 272, 24475–24479 [DOI] [PubMed] [Google Scholar]
- 61.Iseli T. J., Walter M., van Denderen B. J., Katsis F., Witters L. A., Kemp B. E., Michell B. J., Stapleton D. (2005) J. Biol. Chem. 280, 13395–13400 [DOI] [PubMed] [Google Scholar]
- 62.Schulz T. J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. (2007) Cell Metab. 6, 280–293 [DOI] [PubMed] [Google Scholar]
- 63.Hawley S. A., Ross F. A., Chevtzoff C., Green K. A., Evans A., Fogarty S., Towler M. C., Brown L. J., Ogunbayo O. A., Evans A. M., Hardie D. G. (2010) Cell Metab. 11, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young L. H. (2008) Circulation 117, 832–840 [DOI] [PubMed] [Google Scholar]
- 65.Kudo N., Barr A. J., Barr R. L., Desai S., Lopaschuk G. D. (1995) J. Biol. Chem. 270, 17513–17520 [DOI] [PubMed] [Google Scholar]
- 66.Murphy E., Steenbergen C. (2008) Physiol. Rev. 88, 581–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell R. R., 3rd, Li J., Coven D. L., Pypaert M., Zechner C., Palmeri M., Giordano F. J., Mu J., Birnbaum M. J., Young L. H. (2004) J. Clin. Invest. 114, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sukhodub A., Jovanović S., Du Q., Budas G., Clelland A. K., Shen M., Sakamoto K., Tian R., Jovanović A. (2007) J. Cell. Physiol. 210, 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.