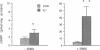Abstract
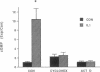
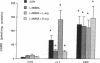
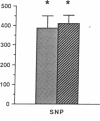
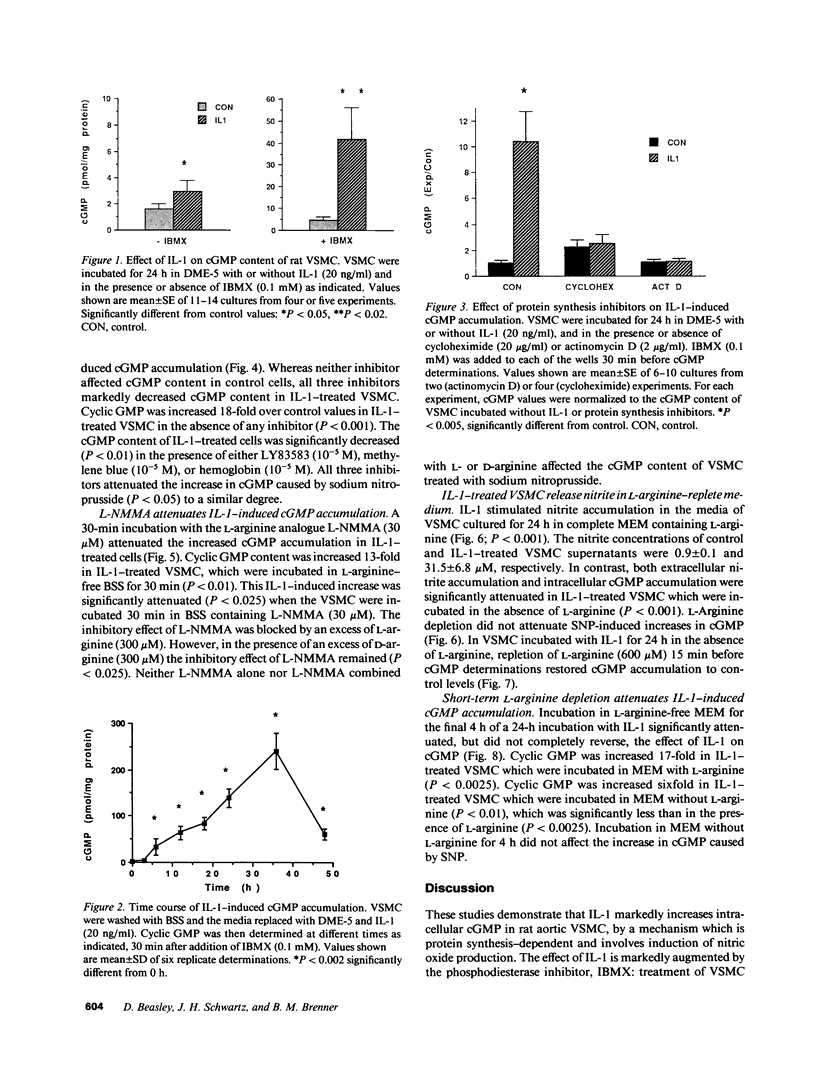
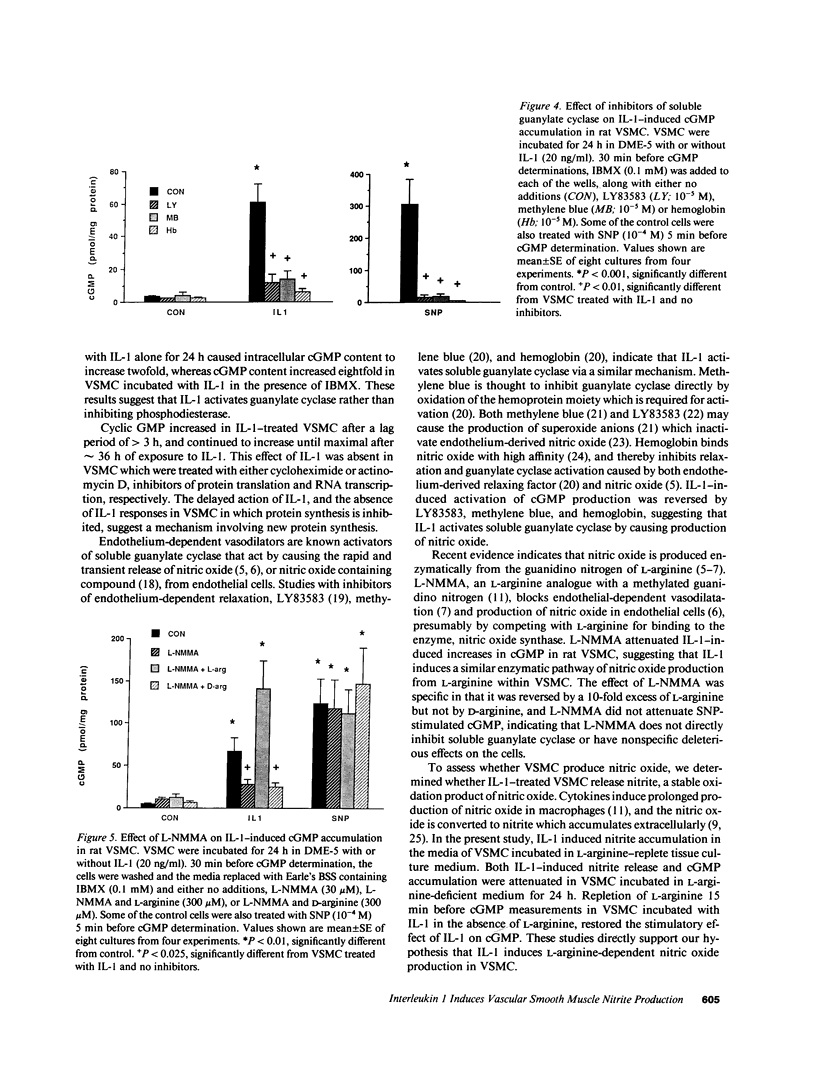
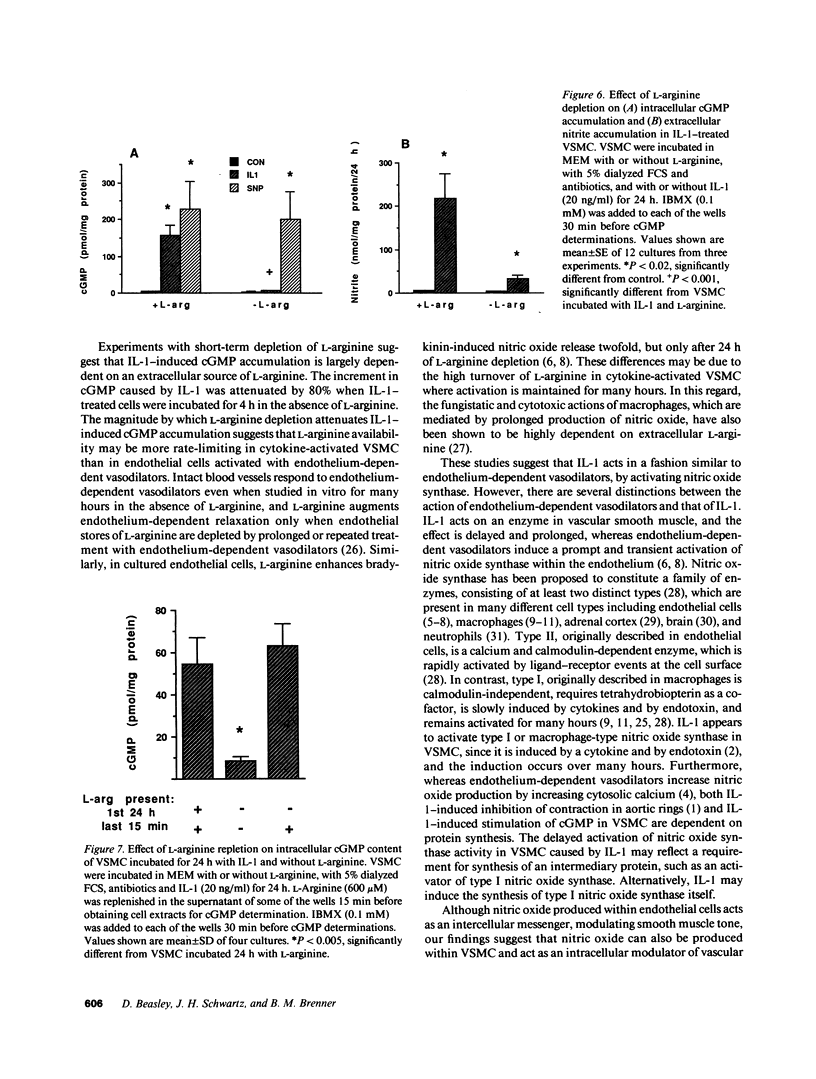
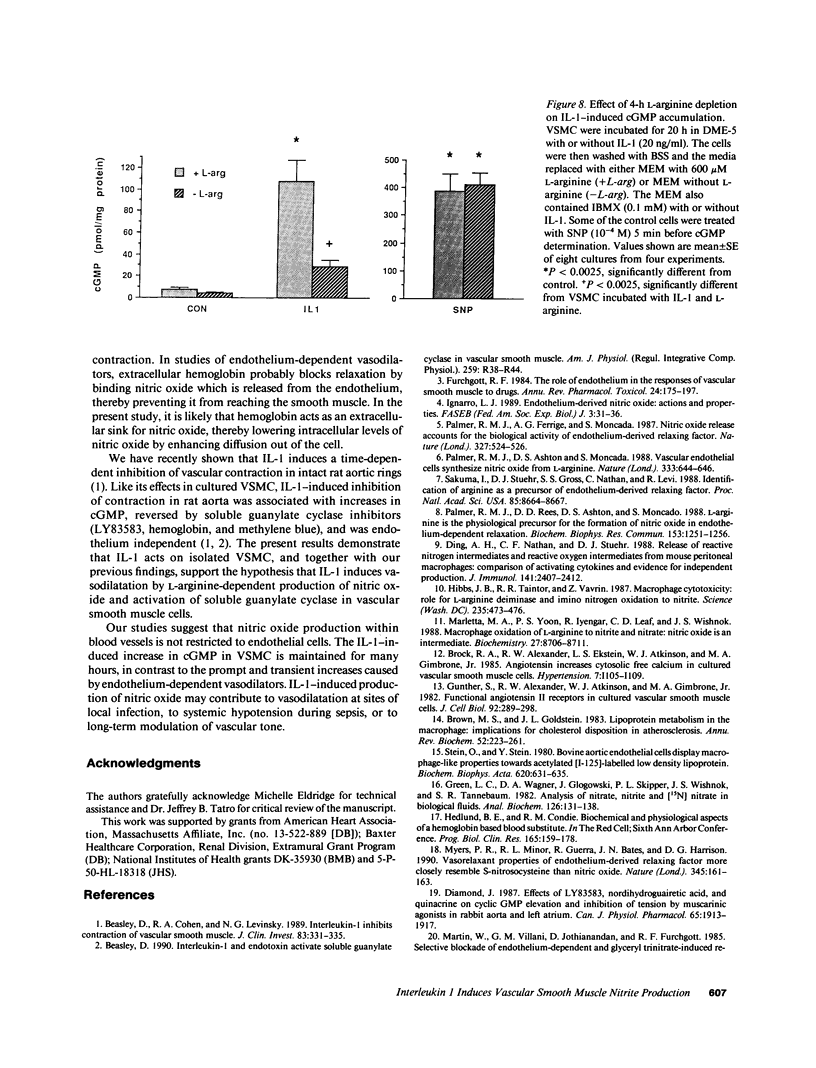
The cytokine interleukin 1 (IL-1) inhibits contractile responses in rat aorta by causing endothelium-independent and prolonged activation of soluble guanylate cyclase. The present study tested whether IL-1 activates guanylate cyclase by inducing prolonged production of nitric oxide in cultured rat aortic vascular smooth muscle cells (VSMC). IL-1 induced a marked time-dependent increase in cyclic guanosine monophosphate (cGMP) in VSMC which was significant at 6 h, and increased progressively for up to 36 h. This effect of IL-1 was abolished when protein synthesis was inhibited with cycloheximide or actinomycin D, suggesting that the effect of IL-1 involves new protein synthesis. IL-1-induced cGMP accumulation was inhibited by the soluble guanylate cyclase inhibitors, methylene blue, LY83583, and hemoglobin and by the L-arginine analogue NGmonomethyl-L-arginine (L-NMMA). The inhibitory effect of L-NMMA was reversed by a 10-fold excess of L-arginine, but not by D-arginine. Nitrite, an oxidation product of nitric oxide, accumulated in the media of VSMC incubated with IL-1 for 24 h in the presence of L-arginine, whereas both IL-1-induced cGMP accumulation and nitrite production were attenuated in VSMC incubated in L-arginine-deficient medium. In L-arginine-depleted VSMC, IL-1-induced cGMP accumulation was restored to control levels by a 15-min incubation with L-arginine. These results demonstrate that IL-1 activates guanylate cyclase in rat VSMC by inducing production of nitric oxide via a pathway dependent on extracellular L-arginine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley D., Cohen R. A., Levinsky N. G. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989 Jan;83(1):331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D. Interleukin 1 and endotoxin activate soluble guanylate cyclase in vascular smooth muscle. Am J Physiol. 1990 Jul;259(1 Pt 2):R38–R44. doi: 10.1152/ajpregu.1990.259.1.R38. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Diamond J. Effects of LY83583, nordihydroguaiaretic acid, and quinacrine on cyclic GMP elevation and inhibition of tension by muscarinic agonists in rabbit aorta and left atrium. Can J Physiol Pharmacol. 1987 Sep;65(9):1913–1917. doi: 10.1139/y87-297. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957 May 23;136(3):507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. E., Bush P. A., Ignarro L. J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund B. E., Condie R. M. Biochemical and physiological aspects of a hemoglobin based blood substitute. Prog Clin Biol Res. 1984;165:159–178. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970 Mar 25;245(6):1374–1377. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Lückhoff A., Pohl U., Busse R., Bassenge E. LY 83583 (6-anilino-5,8-quinolinedione) blocks nitrovasodilator-induced cyclic GMP increases and inhibition of platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):119–125. doi: 10.1007/BF00169217. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Stuehr D. J. Does endothelium-derived nitric oxide have a role in cytokine-induced hypotension? J Natl Cancer Inst. 1990 May 2;82(9):726–728. doi: 10.1093/jnci/82.9.726. [DOI] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Wilke P., Evers B., Böhme E. Enzymatic formation of nitrogen oxides from L-arginine in bovine brain cytosol. Biochem Biophys Res Commun. 1989 Nov 30;165(1):284–291. doi: 10.1016/0006-291x(89)91067-x. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Bovine aortic endothelial cells display macrophage-like properties towards acetylated 125I-labelled low density lipoprotein. Biochim Biophys Acta. 1980 Dec 5;620(3):631–635. doi: 10.1016/0005-2760(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]