Abstract
The Ser/Thr specific-phosphatase PHLPP (Pleckstrin Homology (PH) domain leucine-rich repeat protein phosphatase) provides “the brakes” for Akt and protein kinase C (PKC) signaling. The two isoforms of this recently discovered family, PHLPP1 and PHLPP2, control the amplitude and duration of signaling of Akt and PKC by catalyzing the dephosphorylation of the hydrophobic phosphorylation motif, a C-terminal phosphorylation switch that controls the activity of these kinases. Aberrant regulation of either kinase accompanies many diseases, notably diabetes and cancer. By specifically dephosphorylating the hydrophobic motif, PHLPP controls the degree of agonist-evoked signaling by Akt and the cellular levels of PKC. This review focuses on the function of PHLPP1 and PHLPP2 in modulating signaling by Akt and PKC.
Introduction
Akt and PKC transduce the myriad of cellular signals relayed by lipid second messengers [1–4]. Akt is activated by 3′-phosphoinositides generated following mitogen-mediated activation of phosphatidylinositol 3-kinase (PI3K), and PKC is activated by the lipid second messenger diacylglycerol generated following agonist-evoked activation of phospholipase C (see Boxes 1 and 2). Both kinases require priming phosphorylation by the same upstream kinase, phosphoinositide-dependent kinase-1, PDK-1 [5]. In the case of Akt, this phosphorylation is agonist-evoked and in the case of PKC, it is constitutive. Phosphorylation by PDK-1 triggers a second phosphorylation on a conserved segment on the C-terminal tail of the kinases, named the hydrophobic motif. Phosphorylation of this site depends on the mTORC2 complex [6–8]. A third phosphorylation site, the turn motif, is constitutively phosphorylated on both kinases. Thus, lipid second messengers and phosphorylation play key roles in propagating signals by Akt and PKC.
Box 1. Akt Activation and Signaling.
Activation of Akt is dependent upon PI3K generating the lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) which provides a docking site for proteins that contain PH domains, such as Akt [4]. Growth factor stimulation of tyrosine kinase receptors results in activation of PI3K and production of the lipid second messenger PIP3 [49]. PIP3 binds to the PH domain of Akt, resulting in a ‘PH-out’ conformation so that constitutively bound PDK-1 can phosphorylate Akt at the activation loop (Thr308 in Akt1) [50]. This is accompanied by phosphorylation of Akt at the hydrophobic motif, (Ser473 in Akt1), an event that requires functional mTORC2, a complex composed of rictor, mLST8, mSin1 variants, and the mTOR kinase [6–8, 51].
Akt is a crucial orchestrator of proliferation, cell survival, and cell growth and aberrant activation of Akt results in unregulated cell growth, proliferation, and inhibition of apoptosis, hallmarks of the tumorigenic process. (for a detailed review see Manning and Cantley [3]). Akt phosphorylates the cell cycle inhibitors p21 and p27 on a Thr residue located in the nuclear localization signal to promote cytosolic sequestration of these cell cycle inhibitors and progression through the cell cycle [52–55]. Another Akt substrate involved in cell cycle regulation is the E3 ubiquitin ligase HDM2 [56]. Akt phosphorylates HDM2 to promote its nuclear import, and once present in the nucleus, HDM2 binds to p53 and inhibits p53-mediated transcription. HDM2 bound to p53 also promotes the nuclear export of p53 and promotes its proteasome-mediated degradation, ultimately leading to progression through the cell cycle and inhibition of apoptosis [56]. Akt regulates cell survival through phosphorylation of members of the Forkhead Box O (FoxO) family of transcription factors (including FoxO1, FoxO3A and FoxO4) [3, 57]. Another mediator of cell survival regulated by Akt is glycogen synthase kinase-3; phosphorylation of this substrate results in inhibition of kinase activity, leading to inhibition of apoptosis. Akt regulates cell growth through activation of the mTORC1 pathway [58]. Specifically, Akt phosphorylates two proteins that suppress mTORC1 kinase activity, thereby activating mTORC1: tuberin (TSC2) and Proline-rich Akt substrate 40 kDa (PRAS40) [59, 60].
Box 2. Mechanisms of PKC activation.
PKC, like Akt, is a member of the AGC family of kinases. There are 10 PKC family members in mammals and they are grouped into three distinct subfamilies based on their domain structure, which in turn dictates their regulation by second messengers. Conventional PKC isozymes (α, β1, βII, γ) are activated by diacylglycerol and Ca2+ through their C1 and C2 domains, respectively [1]; these two domains serve as membrane-targeting modules. Specifically, binding of Ca2+ to the C2 domain causes the domain to bind membranes, thus pre-targeting PKC to the membrane where it can bind its membrane-embedded activating ligand, diacylglycerol via the C1 domain. Novel PKC isoforms are activated by diacylglycerol alone (their C1 domain binds this lipid second messenger an order of magnitude more tightly than that of the conventional PKCs). Atypical PKCs do not have a diacylglycerol-sensitive C1 domain or C2 domain and are thus not activated by diacylglycerol or Ca2+.
All PKC family members are processed by a series of ordered phosphorylations before they are catalytically-competent [5]. This processing has been best described for the conventional PKC, PKC βII. The first phosphorylation on this isozyme is catalyzed by PDK-1 and occurs on the activation loop (Thr500); this triggers two intramolecular autophosphorylations on two conserved residues in the C-terminal tail, the turn motif (Thr 450) and the hydrophobic motif (Ser660). Note that the atypical PKCs have a Glu at the phospho-acceptor position of the hydrophobic motif and thus are processed by two, not three, phosphorylations. This mature (i.e. phosphorylated at activation loop, turn motif, and hydrophobic motif) species of PKC is maintained in an inactive conformation by an autoinhibitory pseudosubstrate sequence that blocks the active site. This autoinhibition is relieved when the membrane binding modules are engaged on the membrane. Thus, unlike Akt, PKC (under most conditions) must be bound to its lipid second messenger to be active in the cell. Thus, PKC activity is acutely terminated by metabolism of diacylglycerol.
Phosphorylation does play a key role, however, in terminating the life cycle of PKC. The active conformation of PKC is highly sensitive to dephosphorylation. Once dephosphorylated, PKC is shunted to degradation. Thus, phosphorylation protects PKC from degradation.
A key role played by members of the PKC family is regulation of cell proliferation and cell survival. Isozymes such as PKC ε are pro-survival whereas others such as PKC δ are typically considered pro-apoptotic [61, 62] although this isozyme is anti-apoptotic is some systems. Thus, PKC is emerging as a major drug target for cancer therapy [63, 64]. It is also a major target in diabetes; for example atypical PKC isozymes play key roles in glucose homeostasis and insulin signaling [65].
Signaling by Akt and PKC is terminated following removal of the lipid second messengers and dephosphorylation of the kinases. Akt signaling is acutely terminated by dephosphorylation (once phosphorylated, activity no longer depends on lipid second messengers). In contrast, PKC is only active when bound to diacylglycerol, thus signaling of PKC is acutely terminated by removal of this lipid second messenger. For this kinase, dephosphorylation serves as a signal to promote the degradation of the kinase, ultimately terminating the life cycle of PKC. Whereas the mechanisms of activation of both kinases are understood in considerable detail, the mechanisms of inactivation are less clear. Identification of the phosphatases that directly dephosphorylate Akt and PKC could serve as major therapeutic targets; inhibition of such phosphatases would potentially promote Akt signaling and have ramifications for heart disease and diabetes, whereas activation of such phosphatases could prevent Akt activation and reduce PKC levels, thereby having potential for cancer therapy.
Discovery of the PHLPP Phosphatases
The discovery of the PHLPP family of phosphatases is an example of Pasteur’s aphorism that ‘chance favors the prepared mind’. Reasoning that the phosphatase that terminates Akt signaling may have a PH domain because many of the players in lipid second messenger signaling have such a module (notably the upstream kinase PDK-1 and Akt itself), a query of the NCBI database for genes predicted to encode a PH domain and a phosphatase domain revealed the existence of precisely one such gene family. (Ironically, the PH domain, added late in evolution, is dispensible for Akt (but not PKC) regulation.) The protein family was named after its domain composition: PHLPP (pronounced ‘flip’) for PH domain Leucine-rich repeat Protein Phosphatase [9]. A differential display method had previously identified an mRNA that oscillated in a circadian rhythm-dependent manner in the suprachiasmatic nucleus (SCN); the predicted protein was named SCOP for SCN circadian oscillatory protein and corresponds to one of the splice variants of PHLPP, PHLPP1β [10] (see below).
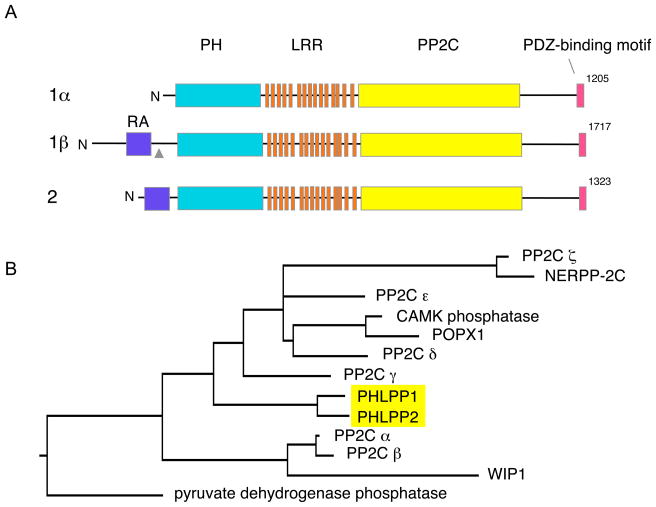
The PHLPP family of phosphatases comprises three members: PHLPP1α (1205 amino acids), PHLPP1β (1717 amino acids), and PHLPP2 (1323 amino acids) [9, 11] (Figure 1A). PHLPP1α and PHLPP1β are splice variants from the same gene, located at chromosome 18q21.33 [11]. These two isoforms are generated from two unique transcripts rather than from alternative initiation of translation start sites on the same transcript. PHLPP1β differs from PHLPP1α by an approximately 56 kDa N-terminal extension (Figure 1A). The PHLPP2 gene resides at the chromosomal location 16q22.3 [11]. PHLPP1 and PHLPP2 possess an identical domain structure with a PH domain followed by region of leucine-rich repeats (LRR), a PP2C phosphatase domain, and a C-terminal PDZ ligand (Figure 1). In addition, PHLPP1β and PHLPP2 contain a Ras association domain (RA domain) preceding the PH domain. PHLPP1 and PHLPP2 share 58% and 63% amino identity in the PP2C domain and PH domain, respectively [9, 11]. SCOP, cloned by Shimizu and colleagues from both the rat and human [10] corresponds to PHLPP1β.
Figure 1.
Domain composition and phylogeny of PHLPP isoforms
A. Domain composition of PHLPP family members showing the Ras association domain (RA; dark blue), pleckstrin homology domain (PH; cyan), Leucine rich repeat (LRR; orange) region, PP2C domain (yellow), and PDZ binding motif (pink). Gray arrow head denotes the splice site for PHLPP1β. B. Phylogenetic tree of PPM phosphatase domains (human) showing PHLPP as a discrete subfamily (highlighted in yellow) of the PP2C class. Sequences were aligned using ClustalW (Gonnet matrix)
The PHLPP1 and PHLPP2 genes reside at chromosomal locations frequently lost in various cancers. The region that includes the PHLPP1 gene undergoes loss of heterozygosity (LOH) in a high percentage of colon cancers [12]. This region, 18q21.33, has several candidate tumor suppressors such as Maspin and BCL2 [13]. LOH has also been observed at the PHLPP2 locus in breast and ovarian cancers, Wilms tumors, prostate cancer, and hepatocellular carcinomas [14–18]; in addition to PHLPP2, the 16q22.3-16q23.1 region harbors potential tumor suppressors such as Breast cancer anti-estrogen resistance 1 (BCAR1) [14]. Additionally the PHLPP2 gene spans a chromosomal region that includes a fragile site (FRA16B); such sites are specific chromosomal regions that display breaks or gaps. These observations suggest that both PHLPP1 (PHLPP1α and PHLPP1β) and PHLPP2 could be candidate tumor suppressor genes, as discussed below. Consistent with this, Pardee and coworkers have recently shown that metastatic breast cancer cells have decreased levels of PHLPP and increased levels of Ser473 phosphorylation [19].
Cellular localization studies reveal that both PHLPP1 and PHLPP2 are present in the cytosolic, nuclear, and membrane fraction of cells [11]. Both isoforms are expressed in numerous cell lines examined, including brain, breast, lung, prostate, and ovarian cancer cell lines [9, 11]. Consistent with this, both isoforms are expressed in the majority of human tissues (NCBI Unigene and GNF online tissue databases).
PHLPP: a Novel PP2C-type Phosphatase Family
There are three main families of Ser/Thr phosphatases: Protein Phosphatase Mg2+ -activated (PPM), Protein Phosphatase P (PPP), and TFIF-associating component of CTD phosphatase (FCP) [20]. PHLPP is a member of the PPM subfamily of phosphatases: it requires Mg+2 or Mn+2 for its catalytic activity and is not inhibited by traditional phosphatase inhibitors such as okadaic acid [9, 11]; these characteristics are hallmarks of PPM type phosphatases [20]. The PPM family of phosphatases includes the PP2C phosphatases (e.g. PP2Cα and PP2Cβ) and pyruvate dehydrogenase phosphatase [21]. Phylogenetic analysis of the phosphatase domains of PPM family members reveals that PHLPP isoforms constitute a discreet subfamily within the PP2C phosphatase family (Figure 1B). The catalytic activity of PPM-type phosphatases requires binding of two Mg+2 or Mn+2 ions, which allows an associated water molecule to act as a nucleophile that can hydrolyze the phosphomonoester bond of a substrate phosphorylated on Ser or Thr residues [20]. The PHLPP PP2C domain contains two of the four invariant aspartic acid residues required for binding to these divalent cations (Table I, invariant aspartic acid residues are boxed in yellow) as well as an invariant basic residue that coordinates the phosphate (Table 1, boxed in cyan) [21]. Curiously, two of the metal-coordinating Asp appear to be absent in the PHLPP family; indeed one is replaced by a Lys. Perhaps the activity of this phosphatase has been tuned to a lower level because it is scaffolded with its substrates. Most PP2C family members comprise a phosphatase domain alone, although some do possess other functional domains, notably PP2Cγ which has a collagen-binding domain, and pyruvate dehydrogenase phosphatase which contains a Ca+2 binding domain [20].
Table 1.
Alignment of invariant metal-coordinating aspartic acid residues in motifs 1, 2, 8, and 11 of PP2C family members*.
 |
The highlighted Asps in motifs 1, 2, 8, and 11 correspond to amino acids 38, 60, 239 and 282, respectively, in PP2Cα; the highlighted Arg in motif 1 corresponds to amino acid 33. Alignment of PP2C sequences of the indicated phosphatases was performed using the PP2C model in Pfam 22.0 [http://pfam.janelia.org].
Domain Structure of the Regulatory Moieties
PHLPP is unique amongst PP2C family members in having multiple domains (RA, PH, LRR, PDZ binding motif) associated with signaling. The N-terminal RA domain in PHLPP1β and PHLPP2 is conserved from yeast to humans. It remains to be determined whether this domain in PHLPP binds to Ras, but PHLPP1β (SCOP) was reported to bind to Ras in lipid rafts via the LRR [22]. The LRR region is found between the PH domain and the PP2C domain. The PH domain is present in all PHLPP isoforms. It is dispensable for the cellular regulation of Akt, but required for the cellular regulation of PKC. A PHLPP1α construct lacking the PH domain fails to dephosphorylate PKC at the hydrophobic motif [23] but does not alter PHLPP1α’s ability to regulate Akt phosphorylation or induce apoptosis [9]. The PH domain of both PHLPP1 and PHLPP2 contains only the middle Arg in the R-X-R-X-F motif required to bind phosphoinositides and it remains to be established whether the module binds phosphoinositides.
The PHLPP phosphatases contain type I PDZ binding motifs at their C-termini [24]. The motifs for PHLPP1 and PHLPP2 are DTPL and DTAL, respectively. The PDZ binding motif in PHLPP1α is critical to the regulation of Akt hydrophobic motif dephosphorylation, regulation of apoptosis, and tumor suppressor function [9]. Expression of a PHLPP1α construct lacking the PDZ binding motif impairs its ability to dephosphorylate Akt, but not PKC [9, 23]. The observation that deletion of the PDZ binding motif impairs PHLPP’s ability to regulate apoptosis and suppress tumor growth, suggests Akt is a critical substrate for these functions [9]. The requirement for the PDZ binding motif suggests that scaffolding of PHLPP to specific signaling complexes within the cell controls its function. Indeed, this is the case for the lipid phosphatase PTEN that terminates PI3-kinase signaling: the PDZ binding motif of PTEN is required to fully suppress the activation of Akt [25].
Evolutionary Conservation of PHLPP
PHLPP is an evolutionarily conserved phosphatase with a homologue in yeast termed CYR1 (NCBI, homologene database). The yeast homologue has a RA domain, leucine rich repeats, and PP2C phosphatase domain. Interestingly, the C-terminus of CYR1 has a second signaling enzyme: an adenylate cyclase domain that is present only in the Ascomycota phylum (which includes Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Neurospora crassa). The link between PHLPP and cAMP in yeast provides a tantalizing suggestion that PHLPP may control cAMP signaling in mammals. The PH domain of PHLPP was an addition that occurred later in evolution, and is present in amniotes, members of a class of tetrapod vertebrates that includes mammals. The PDZ binding motif is present in Bilateria phylum (animals having a bilateral symmetry). PHLPP1 and PHLPP2 branched off in the Euteleostomi clade that includes 90% of living vertebrates.
Targets of PHLPP
Akt
PHLPP was discovered in a rational search for a phosphatase that dephosphorylates Akt and, thus, Akt holds the honor of being the first identified substrate of PHLPP [9]. There are three isoforms of Akt (also known as protein kinase B, PKB) in mammals: Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ). All three isoforms require phosphorylation at the activation loop (Thr308 in Akt1) and hydrophobic motif (Ser473 in Akt1) to attain full catalytic activity [4] (see Box 1). Akt phosphorylated at Thr308 only regulates a subset of Akt substrates and additional phosphorylation at Ser473 fully activates Akt to regulate all characterized downstream substrates of Akt [6, 8]. In vitro studies have established that Akt phosphorylated only at Thr308 is 10% as catalytically active as Akt phosphorylated at both Ser473 and Thr308 [8, 26]. Thus phosphorylation of the hydrophobic motif controls both the activity and substrate specificity of Akt.
PHLPP1 and 2 specifically dephosphorylate the hydrophobic motif of Akt in cells, resulting in decreased activity of Akt, increased apoptosis, and inhibition of cell proliferation. As stated above, the biological effects of PHLPP on Akt require the PDZ-binding motif [9]. Most strikingly, expression of wild-type PHLPP1 in glioblastoma cell lines was shown to dramatically reduce tumor size in a nude mouse model, an effect that was lost upon deletion of the PDZ-binding motif. These results underscore the importance of localization modules (in this case the PDZ motif) in cellular signaling networks.
Genetic depletion studies in mammalian cells have revealed that both PHLPP1 and PHLPP2 exert a dramatic suppression of the amplitude and duration of agonist-evoked activation of Akt [11]. Loss of either PHLPP1 or PHLPP2 results in a striking 30-fold increase in the amplitude of Akt phosphorylation following agonist stimulation. There is also a striking increase in the duration of Akt phosphorylation following agonist-induced activation of Akt [11], highlighting the importance of both of these phosphatases in regulating this pivotal kinase that controls both cellular survival and proliferation among other biological functions.
Depletion of endogenous Akt and PHLPP isoforms has identified unique signaling axes, whereby specific PHLPP isoforms regulate the phosphorylation state of specific Akt isoforms; these Akt isoforms in turn regulate the phosphorylation state of specific downstream substrates [11]. Depletion of endogenous PHLPP2 results in an increase in phosphorylation of the following Akt substrates: GSK-3β, TSC2, FoxO, and p27 [11]. Depletion of endogenous PHLPP1 results in an increase in the phosphorylation state of many of the same Akt substrates (e.g. GSK-3β, TSC2, and FoxO), but also increases the phosphorylation state of a unique set of Akt substrates including HDM2 and GSK-3α [11]. This differential control of specific Akt substrates arises from specificity in the regulation of the three Akt isoforms by PHLPP1 and PHLPP2. Genetic depletion studies have revealed that PHLPP1 influences the phosphorylation state of Akt2 and Akt3, whereas PHLPP2 affects the phosphorylation state of Akt1 and Akt3. Co-immunoprecipitation studies support an interaction of PHLPP1 with Akt2 and Akt 3 (but not Akt1) and PHLPP2 with Akt1 and Akt3 (but not Akt2). Thus, there is specific wiring within the cell of PHLPP-Akt-substrate pathways. The most specific ones are: PHLPP1-Akt2-HDM2 and PHLPP2-Akt3-p27. Other substrates such as GSK-3β are controlled by all PHLPP isoforms and all Akt isoforms. Consistent with specific wiring of Akt pathways, a number of reports demonstrate that Akt isoforms can regulate the phosphorylation of specific downstream substrates of Akt. For example, Akt2 was reported to specifically regulate the phosphorylation of GSK-3α [27]. Figure 2 summarizes the ‘wiring’ of PHLPP-Akt-substrates based on genetic depletion experiments in H157 (human non small cell lung cancer cell line) and Hs578Bst (human breast cell line) cells [11].
Figure 2.
Wiring of PHLPP/Akt pathways
PHLPP1 and PHLPP2 selectively control the phosphorylation state of specific Akt isozymes, which in turn control the phosphorylation of specific substrates. Following mitogen stimulation, Akt is phosphorylated on the activation loop (pink; Thr308 in Akt1) by PDK-1, an event that triggers phosphorylation of the hydrophobic motif by a mechanism that depends on mTORC2 (green; Ser473 in Akt1). Akt1, Akt2, and Akt3 phosphorylate both isoform-specific (HDM2, p27) and shared (GSK-3β) substrates. Specific interactions (light blue ovals) of PHLPP1 or PHLPP2 with Akt1, Akt2, or Akt3 allow PHLPP isoforms to differentially control the amplitude of Akt signaling towards downstream substrates. Adapted from [11] with permission.
PKC
The hydrophobic motif is present on PKC isoforms; indeed this phosphorylation motif was simultaneously identified on PKCβII and p70S6kinase in 1995 [28, 29]. Studies with conventional isozymes have revealed that phosphorylation of this site does not significantly affect PKC activity; rather, it controls the stability of the kinase. Dephosphorylation at this position shunts PKC to the detergent-insoluble fraction where it is degraded. Thus, phosphorylation at the hydrophobic motif controls the amplitude of PKC signaling by controlling the amount of PKC in the cell.
Both PHLPP1 and PHLPP2 dephosphorylate the hydrophobic motif of conventional and novel PKC isoforms but not atypical PKC isoforms, because they have a Glu at this position [23]. This dephosphorylation triggers the degradation of PKC. Thus, depletion of PHLPP1 or PHLPP2 results in a robust increase in levels of PKC (the fully-phosphorylated mature species, Box 2) owing to increased stability from phosphorylation on the hydrophobic motif (Ser657 PKCα) [23].
Interestingly, cells deficient in the mTORC2 complex have decreased PKC phosphorylation at the hydrophobic motif, suggesting this complex contributes to phosphorylation of PKC at this site [8]. Although the mechanism has not been elucidated, this poises PHLPP to oppose the mTORC2 complex. Further studies should elaborate on the interplay between these signaling molecules.
Other substrates of PHLPP
Rat PHLPP1β (SCOP) has been reported to negatively regulate the Ras-Raf-MEK-ERK pathway by interacting directly with Ras [22]. Whether PHLPP directly dephosphorylates components of this pathway remains to be explored. Other potential substrates of PHLPP include AGC kinase family members such as p70S6K, SGK or p90RSK, all of which also have hydrophobic phosphorylation motifs. Overexpression studies suggest that PHLPP2 does not regulate either p70S6K or p90RSK [11]; however under these conditions the phosphatase may be mislocalized and unable to regulate potential downstream substrates. Depletion of either PHLPP1 or PHLPP2 results in an increase in p70S6K phosphorylation at the hydrophobic motif, Thr 389 [11], an event that activates a feedback pathway to inhibit Akt phosphorylation [11].
Potential Role of PHLPP in Disease
Cancer
The PI3K-Akt pathway is a central node in determining cellular fate. Hyperactivation of this pathway tips the balance towards cell survival, growth and proliferation, hallmarks of the tumorigenic process [30]. Somatic mutations resulting in constitutive activation of this pathway are well documented and defined as a causal mechanism driving tumorigenesis. This pathway is activated in cancer by a number of mechanisms, including amplification or gain-of-function mutations in upstream receptor protein tyrosine kinases (RPTKs) [31–33], activating mutations in PI3K and Akt [34, 35], and loss-of-function mutations in the regulatory phosphatase PTEN [36]. Given the importance of this pathway in tumorigenesis, the PHLPP phosphatases are poised to play key roles in this process.
In addition, PKC is the receptor for tumor-promoting phorbol esters, strongly implicating this kinase in tumorigenesis [1]. PKC is also somatically mutated in many cancers; however the functional consequences of these mutations have not been determined [37]. Furthermore, PKC levels are grossly altered in many cancers. Providing further rationale that PHLPP phosphatases could be involved in tumorigenesis, both genes are located at chromosomal loci frequently lost in cancer, as highlighted earlier.
Given the specific regulation of Akt isoforms by PHLPP isoforms, it will be important to determine if there is a unique contribution of each PHLPP isoform to the process of tumorigenesis. The role of each Akt isoform in cancer initiation and progression is complex and somewhat contradictory. For example, Akt1 inhibits both invasion and cellular migration in breast cancer cell lines and immortalized MCF-10A cells [38, 39], but Akt1 has also been reported to promote tumorigenesis and metastasis in mice expressing ErbB2 as well as promoting migration in mouse endothelial tumor cells [40]. Consistent with the latter result, Akt1 knockout mice have delayed tumor onset when crossed with mice expressing the oncogenes ErbB2 or polyoma middle T antigen [41]. These studies highlight the complexity of Akt1’s role in cancer and provide evidence that Akt1 can be both a tumor suppressor and an oncogene. Similarly, Akt2 has a complex role in tumorigenesis. Activation of Akt2 leads to increased migration, invasion and metastasis in cell culture systems [39]. Surprisingly Akt2 knockout mice crossed with mice expressing ErbB2 or polyoma middle T antigen have an increased rate of tumor development, suggesting Akt2 may inhibit tumor progression [41]. Given the specific regulation of Akt isoforms by PHLPP1 and PHLPP2, these phosphatases may act as both tumor suppressors and oncogenes.
Unlike PTEN, which is somatically mutated in many cancers, the PHLPP phosphatases are functional in the majority of cancer cell lines examined thus far. This presents a unique opportunity where the PHLPP phosphatases could serve as universal targets for the treatment of cancer. The discovery of small molecule compounds that stabilize these phosphatases would allow inhibition of both PKC and Akt, which could ultimately lead to tumor regression. To this end it has recently been reported that PHLPP2 levels increase 23-fold in rats treated with lanthanum nitrate [42]. Lanthanum-based compounds are known to cause apoptosis and inhibit proliferation in cancer cells [43, 44]; whether these effects are mediated in part by increases in PHLPP2 expression remain to be determined. Given the paradigm shift in drug discovery from specific inhibitors to pan inhibitors that shut down multiple signaling pathways implicated in cancer [45], discovery of a small molecule that activates or increases expression of PHLPP should result in the inactivation of multiple cancer-related signaling pathways.
Metabolic Disorders
One of the primary functions of Akt signaling is to stimulate glucose uptake in response to insulin [3]. Akt2 is the main isoform responsible for glucose uptake, evidenced by the Akt2 knockout mouse that displays a type II diabetic phenotype. Additionally, Akt2 is the main Akt isoform detected in insulin-responsive tissues [46]. Loss of Akt2 results in insulin resistance and hyperglycemia owing to loss of glucose uptake [46]. Consistent with these results, overexpression of an inhibitor of Akt, tribbles, promotes hyperglycemia in mice [47]. Akt stimulates glucose uptake by promoting translocation of the glucose transporter Glut4 to the plasma membrane. Direct inhibtion of PHLPP1 might stimulate Akt2 activity and could be a potential therapeutic target for the treatment of type II diabetes. In this regard, elevated levels of PHLPP1 have been found in skeletal muscle of Type 2 diabetic patients compared to control patients, corresponding specifically to decreased levels of Akt2 phosphorylation [48].
The dephosphorylation catalyzed by the PHLPP isozymes opposes the phosphorylation cascade of PKC and Akt that is triggered by PDK-1 (Figure 3). These isozymes thus serve as “the brakes” for signaling by these two AGC family members. But PHLPP is likely to have many more targets. Given that there are more than 400 Ser/Thr kinases, but fewer than 40 Ser/Thr phosphatases, it is likely that an abundance of additional substrates will be unmasked in the coming years [20]. Exciting challenges lie ahead to decipher the full spectrum of signaling pathways regulated by PHLPP and its role in disease.
Figure 3.
Activation of PKC and Akt pathways by lipid second messengers.
Model showing activation of PKC and Akt signaling pathways by lipid second messengers (diacylglycerol (DG) for PKC and phosphaticylinositol-3,4,5-trisphosphate (PIP3) for Akt) and phosphorylation catalyzed by PDK-1. Phosphorylation by PDK-1 on the activation loop (pink phosphate) triggers phosphorylation on the hydrophobic motif (green phosphate), an event that depends on functional mTORC2 in the cell. Once phosphorylated, Akt moves throughout the cell phosphorylating substrates. PKC, in contrast, must be bound to diacylglycerol to signal. Signaling is terminated by removal of the second messengers (catalyzed by PTEN and diacylglycerol kinase) and removal of the activating phosphates. PHLPP catalyzes the dephosphorylation of the hydrophobic motif, suppressing the amplitude of signaling by Akt and PKC. For Akt, dephosphorylation of the hydrophobic motif acutely inactivates the kinase. For PKC, this chronically controls the levels of the kinase because the dephosphorylated form is rapidly degraded.
Acknowledgments
This work was supported in part by National Institutes of Health R01 GM43154 (ACN), GM067946 (ACN), and DOD BCRP Predoctoral Grant BC043239 (JB). Regarding grant BC043239, the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort DetrickMD21702-5014, is the awarding and administering acquisition office. The content of this article does not necessarily reflect the position or the policy of the U.S.government. We thank Dr. Tianyan Gao and members of the Newton lab for many helpful and enjoyable discussions.
References
- 1.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101(8):2353–64. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 2.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7(4):281–94. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26(11):657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 5.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370(Pt 2):361–71. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacinto E, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127(1):125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, et al. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458(3):363–9. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- 11.Brognard J, et al. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25(6):917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63(7):1608–14. [PubMed] [Google Scholar]
- 13.Johnson-Pais TL, et al. Determination of a minimal region of loss of heterozygosity on chromosome 18q21.33 in osteosarcoma. Int J Cancer. 2003;105(2):285–8. doi: 10.1002/ijc.11070. [DOI] [PubMed] [Google Scholar]
- 14.Rakha EA, et al. Chromosome 16 tumor-suppressor genes in breast cancer. Genes Chromosomes Cancer. 2006;45(6):527–35. doi: 10.1002/gcc.20318. [DOI] [PubMed] [Google Scholar]
- 15.Safford SD, et al. Fine mapping of Wilms’ tumors with 16q loss of heterozygosity localizes the putative tumor suppressor gene to a region of 6.7 megabases. Ann Surg Oncol. 2003;10(2):136–43. doi: 10.1245/aso.2003.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Torring N, et al. Genome-wide analysis of allelic imbalance in prostate cancer using the Affymetrix 50K SNP mapping array. Br J Cancer. 2007;96(3):499–506. doi: 10.1038/sj.bjc.6603476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patael-Karasik Y, et al. Comparative genomic hybridization in inherited and sporadic ovarian tumors in Israel. Cancer Genet Cytogenet. 2000;121(1):26–32. doi: 10.1016/s0165-4608(00)00224-7. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda H, et al. Allele loss on chromosome 16 associated with progression of human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87(17):6791–4. doi: 10.1073/pnas.87.17.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao M, Iglehart JD, Pardee AB. Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer Res. 2007;67(11):5293–9. doi: 10.1158/0008-5472.CAN-07-0877. [DOI] [PubMed] [Google Scholar]
- 20.Cohen PTW. Overview of protein serine/threonine phosphatases. Topics in Current Genetics. 2003;5:1–20. [Google Scholar]
- 21.Shinri Tamura MGL, Komaki Ken-ichiro, Sasaki Masato, Kobayashi Takayasu. Roles of mammalian protein phosphatase 2C family members in the regulation of cellular functions. Topics in Current Genetics. 2003;5:91–105. [Google Scholar]
- 22.Shimizu K, et al. Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J Biol Chem. 2003;278(17):14920–5. doi: 10.1074/jbc.M213214200. [DOI] [PubMed] [Google Scholar]
- 23.Gao T, Brognard J, Newton AC. The Phosphatase PHLPP Controls the Cellular Levels of Protein Kinase C. J Biol Chem. 2008;283(10):6300–11. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 24.Dev KK. Making protein interactions druggable: targeting PDZ domains. Nat Rev Drug Discov. 2004;3(12):1047–56. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci U S A. 2000;97(8):4233–8. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15(23):6541–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang ZY, et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100(13):7569–74. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5(12):1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 29.Pearson RB, et al. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Embo J. 1995;14(21):5279–87. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 31.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 32.Siegel PM, et al. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol Cell Biol. 1994;14(11):7068–77. doi: 10.1128/mcb.14.11.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 34.Carpten JD, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 35.Samuels Y, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7(6):561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 37.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoeli-Lerner M, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20(4):539–50. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Irie HY, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171(6):1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju X, et al. Akt1 governs breast cancer progression in vivo. Proceedings of the National Academy of Sciences. 2007;104(18):7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maroulakou IG, et al. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67(1):167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, et al. Gene expression profiles of hepatocytes treated with La(NO3)3 of rare earth in rats. World J Gastroenterol. 2004;10(11):1625–9. doi: 10.3748/wjg.v10.i11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Y, et al. Effects of rare earth compounds on growth and apoptosis of leukemic cell lines. In Vitro Cell Dev Biol Anim. 2002;38(7):373–5. doi: 10.1290/1071-2690(2002)038<0373:EORECO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Heffeter P, et al. Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24) Biochem Pharmacol. 2007;73(12):1873–86. doi: 10.1016/j.bcp.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33(4):407–20. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35(Pt 2):231–5. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 47.Du K, et al. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300(5625):1574–7. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 48.Cozzone D, et al. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia. 2008;51(3):512–21. doi: 10.1007/s00125-007-0913-8. [DOI] [PubMed] [Google Scholar]
- 49.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 50.Calleja V, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5(4):e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frias MA, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16(18):1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Liang J, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8(10):1153–60. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 53.Shin I, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–52. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 54.Viglietto G, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8(10):1136–44. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 55.Zhou BP, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3(3):245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 56.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98(20):11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112(8):1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10(1):151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 60.Vander Haar E, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9(3):316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 61.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420(2):246–54. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka Y, et al. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278(36):33753–62. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 63.Hofmann J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr Cancer Drug Targets. 2004;4(2):125–46. doi: 10.2174/1568009043481579. [DOI] [PubMed] [Google Scholar]
- 64.O’Brian CA, et al. Protein kinase Calpha and epsilon small-molecule targeted therapeutics: a new roadmap to two Holy Grails in drug discovery? Expert Rev Anticancer Ther. 2006;6(2):175–86. doi: 10.1586/14737140.6.2.175. [DOI] [PubMed] [Google Scholar]
- 65.Farese RV, et al. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest. 2007;117(8):2289–301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]