Abstract
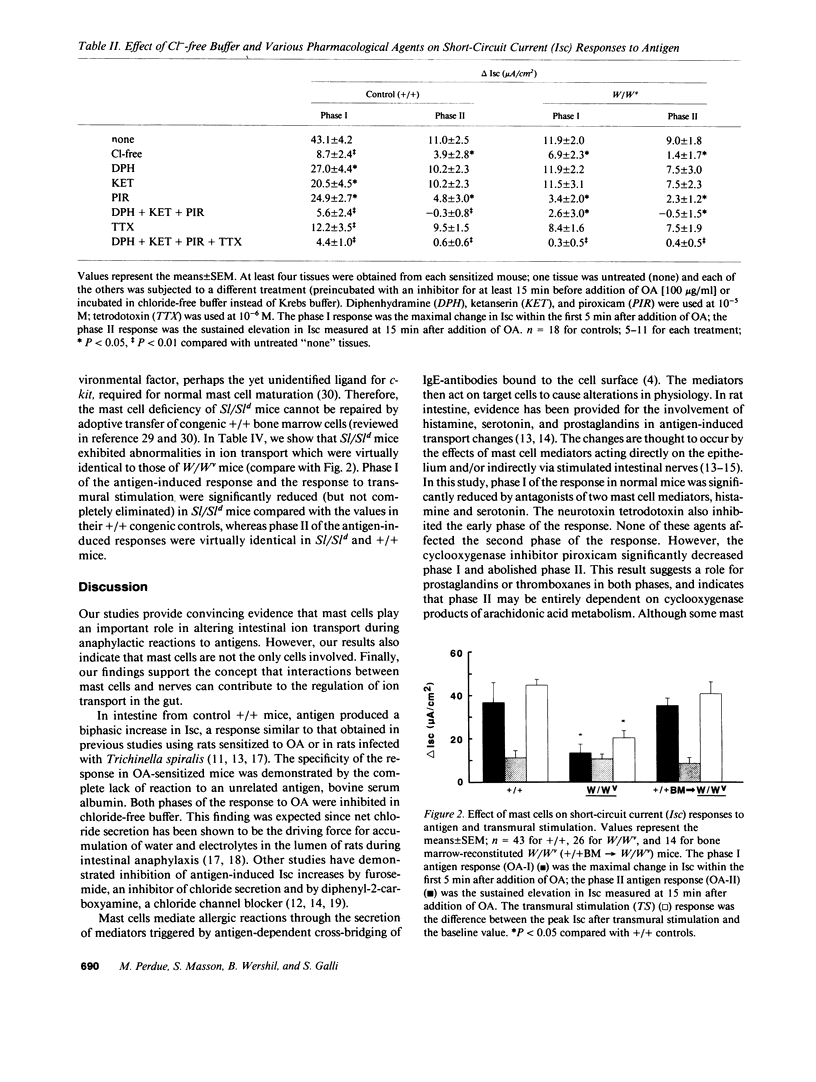
To investigate the role of mast cells in transport abnormalities during intestinal anaphylaxis, we examined responses to antigen in isolated intestinal preparations from ovalbumin-sensitized genetically mast cell-deficient WBB6F1-W/Wv (W/Wv) mice and congenic normal WBBGF1(-)+/+ (+/+) mice. Changes in ion transport (primarily secretion of chloride ions) were indicated by increases in short-circuit current (Isc). In tissues from +/+ mice, antigen caused increases in Isc which were significantly inhibited by antagonists to histamine (diphenhydramine) and serotonin (ketanserin), by a cyclooxygenase inhibitor (piroxicam) and by a neurotoxin (tetrodotoxin). In preparations from W/Wv mice, antigen-stimulated responses were approximately 30% of that in +/+ mice and were inhibited only by piroxicam. Responses to electrical transmural stimulation of nerves were approximately 50% in W/Wv versus +/+ mice, and were inhibited by antagonists of mast cell mediators in +/+ but not W/Wv mice. Reconstitution of mast cells in W/Wv mice by intravenous injection of +/+ bone marrow cells restored the normal responses to both antigen and nerve stimulation. Our results indicate that mast cell-dependent mechanisms are primarily responsible for the ion secretion associated with intestinal anaphylaxis, but that other cells are also involved. In addition, our data provide evidence for the functional importance of bidirectional communication between nerves and mast cells in the regulation of ion transport in the gastrointestinal tract.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arizono N., Matsuda S., Hattori T., Kojima Y., Maeda T., Galli S. J. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990 May;62(5):626–634. [PubMed] [Google Scholar]
- Baird A. W., Cuthbert A. W., MacVinish L. J. Type 1 hypersensitivity reactions in reconstructed tissues using syngeneic cell types. Br J Pharmacol. 1987 Aug;91(4):857–869. doi: 10.1111/j.1476-5381.1987.tb11285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A. W., Cuthbert A. W. Neuronal involvement in type 1 hypersensitivity reactions in gut epithelia. Br J Pharmacol. 1987 Nov;92(3):647–655. doi: 10.1111/j.1476-5381.1987.tb11368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A. W., Cuthbert A. W., Pearce F. L. Immediate hypersensitivity reactions in epithelia from rats infected with Nippostrongylus brasiliensis. Br J Pharmacol. 1985 Aug;85(4):787–795. doi: 10.1111/j.1476-5381.1985.tb11077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani-Sacchi T., Barattini M., Bianchi S., Blandina P., Brunelleschi S., Fantozzi R., Mannaioni P. F., Masini E. The release of histamine by parasympathetic stimulation in guinea-pig auricle and rat ileum. J Physiol. 1986 Feb;371:29–43. doi: 10.1113/jphysiol.1986.sp015960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Sestini P., Perdue M. H. Allergic reactions of rat jejunal mucosa. Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology. 1990 Jul;99(1):74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Jordan C. C., Oehme P., Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983 Feb;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New approaches for the analysis of mast cell maturation, heterogeneity, and function. Fed Proc. 1987 Apr;46(5):1906–1914. [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Chernov T., Renold F., Payan D. G. Neuropeptide regulation of the expression of immediate hypersensitivity. J Immunol. 1985 Aug;135(2 Suppl):802s–805s. [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D., Crowle P. K. Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol. 1986;80(1):85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D. Systemic anaphylaxis in mast-cell-deficient mice of W/Wv and Sl/Sld genotypes. Exp Cell Biol. 1987;55(2):63–68. doi: 10.1159/000163399. [DOI] [PubMed] [Google Scholar]
- Hanglow A. C., Bienenstock J., Perdue M. H. Effects of platelet-activating factor on ion transport in isolated rat jejunum. Am J Physiol. 1989 Nov;257(5 Pt 1):G845–G850. doi: 10.1152/ajpgi.1989.257.5.G845. [DOI] [PubMed] [Google Scholar]
- Harari Y., Russell D. A., Castro G. A. Anaphylaxis-mediated epithelial Cl- secretion and parasite rejection in rat intestine. J Immunol. 1987 Feb 15;138(4):1250–1255. [PubMed] [Google Scholar]
- Heavey D. J., Ernst P. B., Stevens R. L., Befus A. D., Bienenstock J., Austen K. F. Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat intestinal mucosa mast cells. J Immunol. 1988 Mar 15;140(6):1953–1957. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Jacoby W., Cammarata P. V., Findlay S., Pincus S. H. Anaphylaxis in mast cell-deficient mice. J Invest Dermatol. 1984 Oct;83(4):302–304. doi: 10.1111/1523-1747.ep12340431. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kowalski M. L., Kaliner M. A. Neurogenic inflammation, vascular permeability, and mast cells. J Immunol. 1988 Jun 1;140(11):3905–3911. [PubMed] [Google Scholar]
- Lake A. M., Kagey-Sobotka A., Jakubowicz T., Lichtenstein L. M. Histamine release in acute anaphylactic enteropathy of the rat. J Immunol. 1984 Sep;133(3):1529–1534. [PubMed] [Google Scholar]
- MacQueen G., Marshall J., Perdue M., Siegel S., Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989 Jan 6;243(4887):83–85. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- Nawa Y., Owhashi M., Imai J., Abe T. Eosinophil response in mast cell-deficient W/WV mice. Int Arch Allergy Appl Immunol. 1987;83(1):6–11. doi: 10.1159/000234323. [DOI] [PubMed] [Google Scholar]
- Newson B., Dahlström A., Enerbäck L., Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983 Oct;10(2):565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Patrick M. K., Dunn I. J., Buret A., Miller H. R., Huntley J. F., Gibson S., Gall D. G. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988 Jan;94(1):1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Chung M., Gall D. G. Effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology. 1984 Mar;86(3):391–397. [PubMed] [Google Scholar]
- Perdue M. H., Davison J. S. Altered regulation of intestinal ion transport by enteric nerves in diabetic rats. Am J Physiol. 1988 Mar;254(3 Pt 1):G444–G449. doi: 10.1152/ajpgi.1988.254.3.G444. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Intestinal anaphylaxis in the rat: jejunal response to in vitro antigen exposure. Am J Physiol. 1986 Apr;250(4 Pt 1):G427–G431. doi: 10.1152/ajpgi.1986.250.4.G427. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Rat jejunal mucosal response to histamine and anti-histamines in vitro. Comparison with antigen-induced changes during intestinal anaphylaxis. Agents Actions. 1986 Oct;19(1-2):5–9. doi: 10.1007/BF01977249. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Transport abnormalities during intestinal anaphylaxis in the rat: effect of antiallergic agents. J Allergy Clin Immunol. 1985 Sep;76(3):498–503. doi: 10.1016/0091-6749(85)90733-x. [DOI] [PubMed] [Google Scholar]
- Russell D. A. Mast cells in the regulation of intestinal electrolyte transport. Am J Physiol. 1986 Aug;251(2 Pt 1):G253–G262. doi: 10.1152/ajpgi.1986.251.2.G253. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler-Gerard N. P., Galli S. J. Mast cells are not required for anaphylatoxin-induced ileal smooth muscle contraction. J Immunol. 1987 Mar 15;138(6):1908–1913. [PubMed] [Google Scholar]
- Yano H., Wershil B. K., Arizono N., Galli S. J. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989 Oct;84(4):1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]


