Abstract
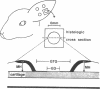
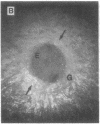
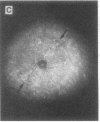
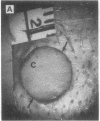
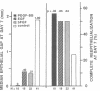
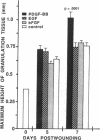
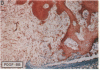
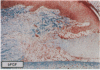
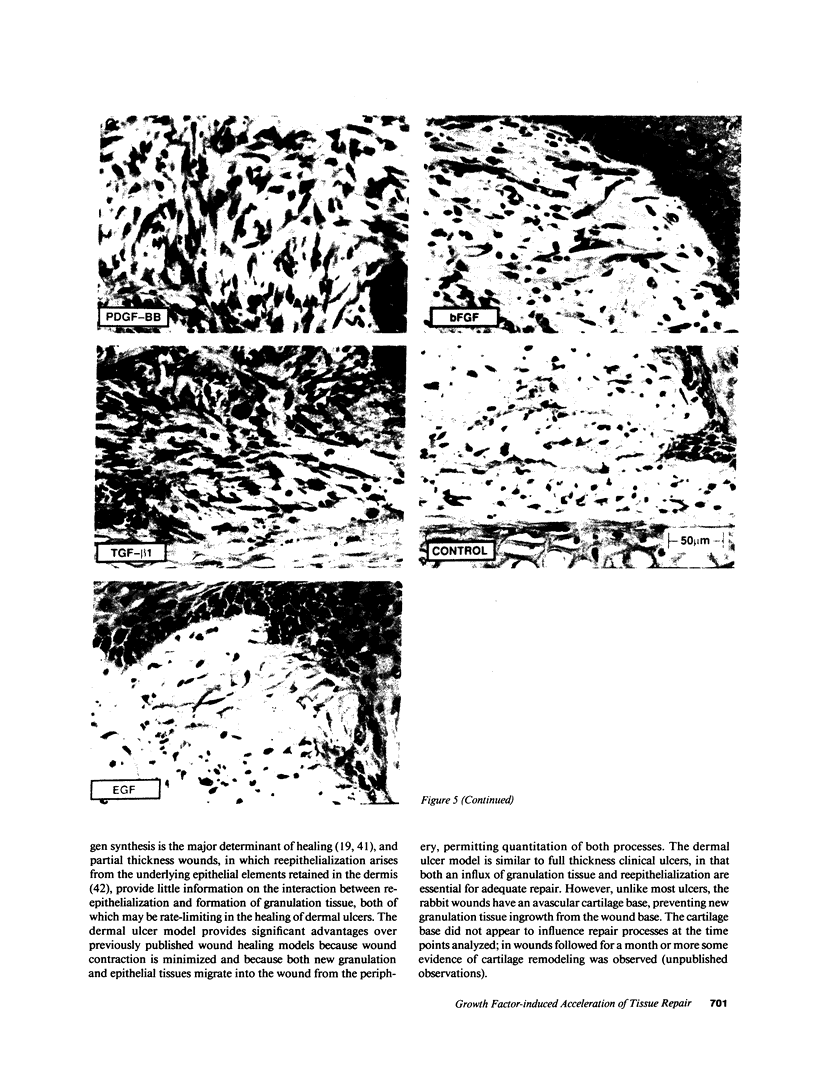
The roles of polypeptide growth factors in promoting wound healing and in directing the specificity and sequence of responses of different tissues in wounds are little understood. We investigated the influence of four growth factors on the rates of healing of a novel full thickness dermal ulcer placed on an avascular base in the rabbit ear. The wound model precludes significant wound contraction and requires new granulation tissue and epithelial cells for healing to originate centripetally. 5 micrograms (7-31 pmol/mm2) of platelet-derived growth factor-B chain (PDGF-BB), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) applied locally at the time of wounding resulted in a twofold increase in complete reepithelialization of treated wounds (PDGF-BB, P = 0.02 chi square analysis; bFGF, P = 0.04; EGF, P = 0.05); transforming growth factor (TGF)-beta 1 significantly inhibited reepithelialization (P = 0.05). Both PDGF-BB and TGF-beta 1 uniquely increased the depth and area of new granulation tissue (P less than 0.005), the influx of fibroblasts, and the deposition of new matrix into wounds. Explants from 7-d old PDGF-BB-treated wounds remained metabolically far more active than controls, incorporating 473% more [3H]thymidine into DNA (P = 0.05) and significantly more [3H]leucine and [3H]proline into collagenase-sensitive protein (P = 0.04). The results establish that polypeptide growth factors have significant and selective positive influences on healing of full thickness ulcers in the rabbit.
Full text
PDF

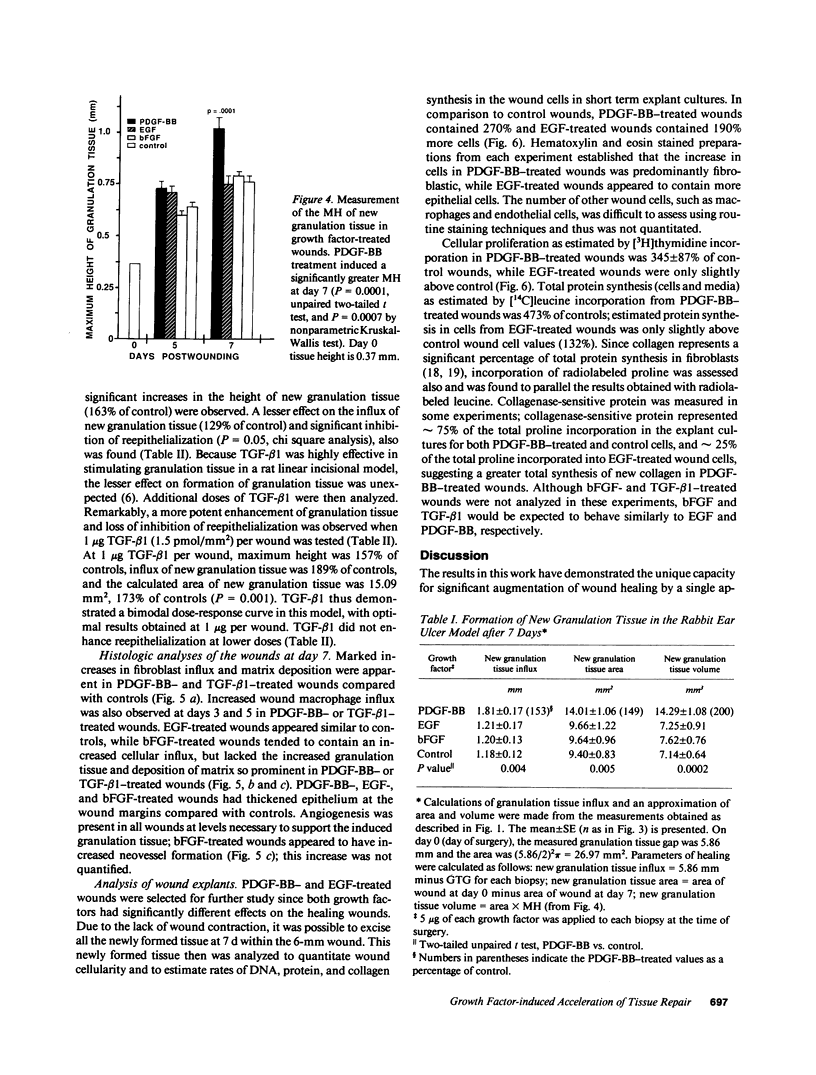
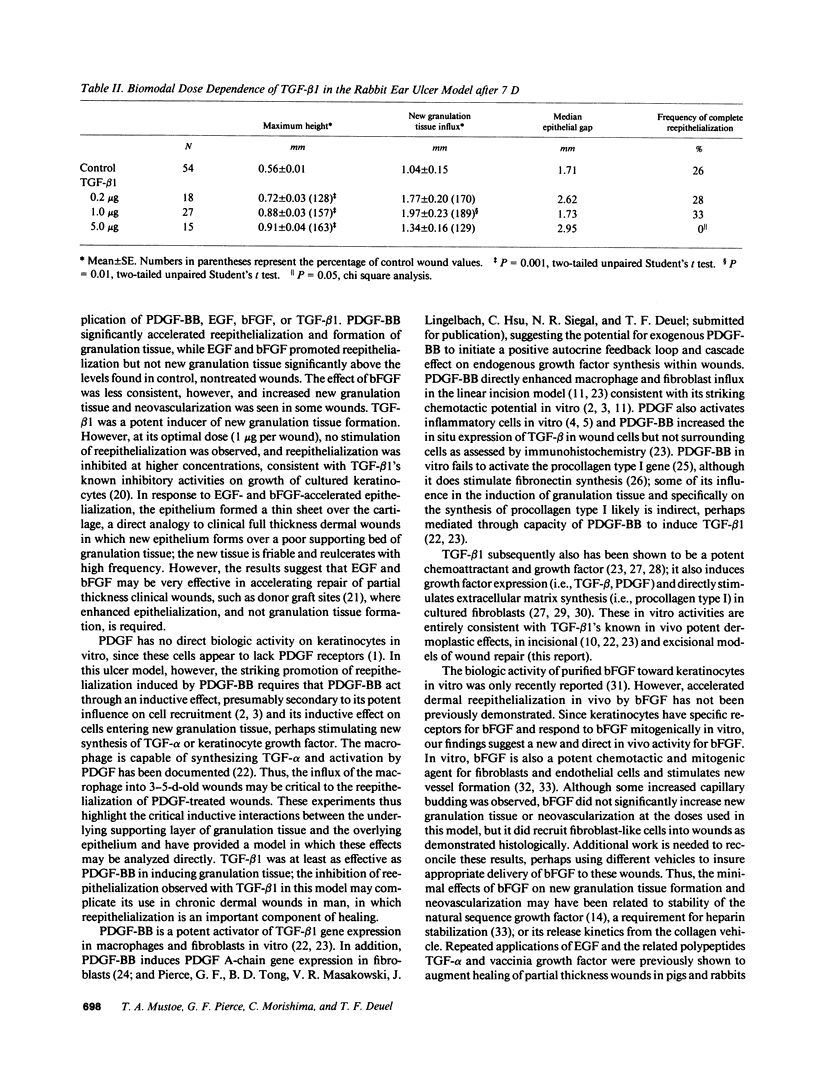
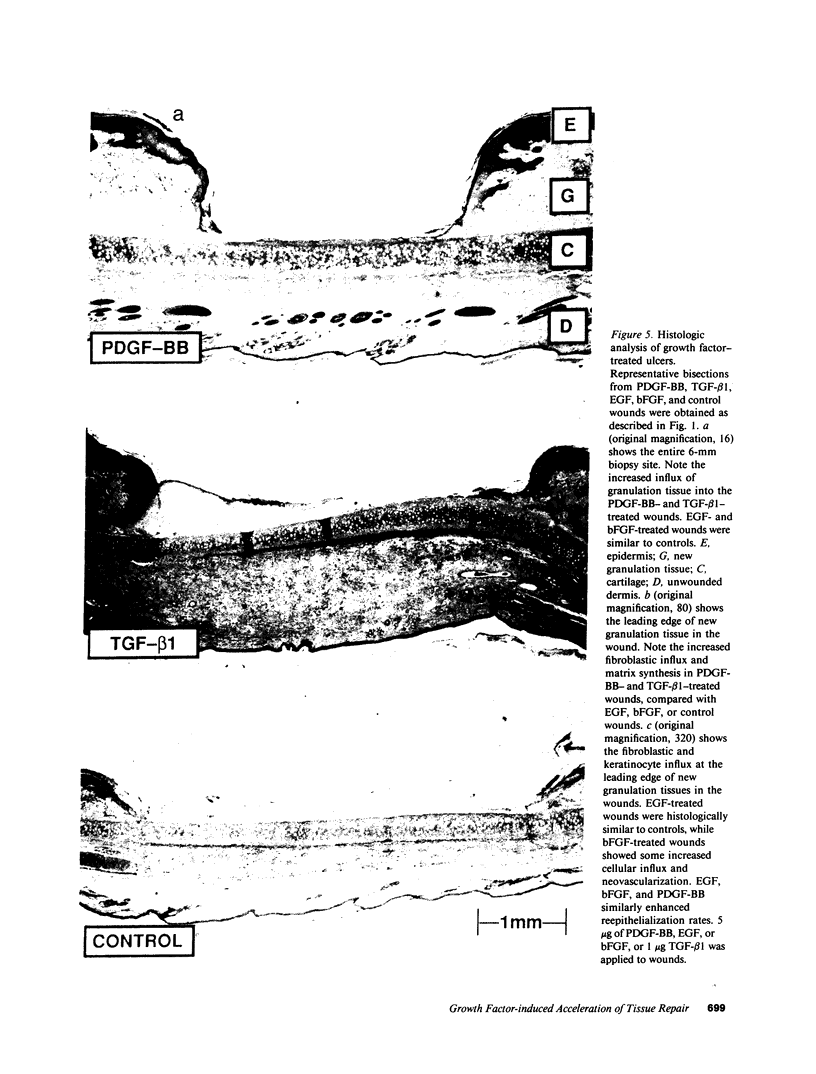
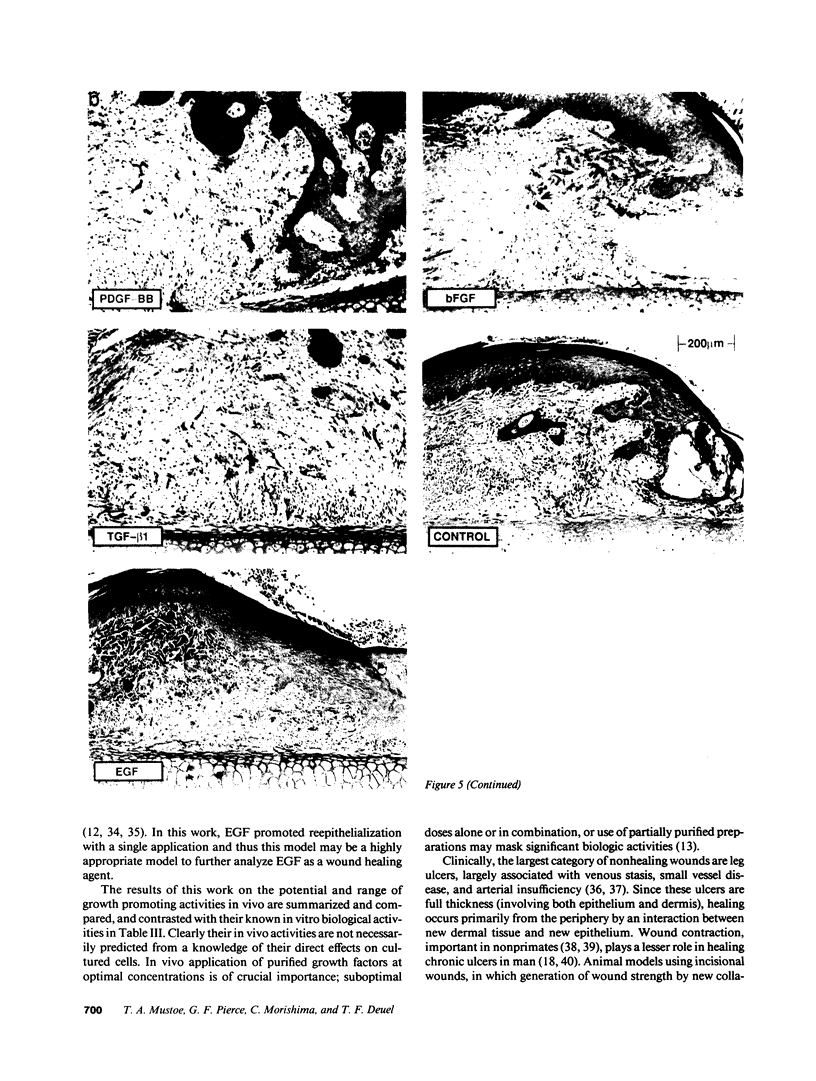


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatti S. P., Foster D. N., Ranganathan G., Moses H. L., Getz M. J. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1119–1123. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- Buckley-Sturrock A., Woodward S. C., Senior R. M., Griffin G. L., Klagsbrun M., Davidson J. M. Differential stimulation of collagenase and chemotactic activity in fibroblasts derived from rat wound repair tissue and human skin by growth factors. J Cell Physiol. 1989 Jan;138(1):70–78. doi: 10.1002/jcp.1041380111. [DOI] [PubMed] [Google Scholar]
- Buckley A., Davidson J. M., Kamerath C. D., Wolt T. B., Woodward S. C. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7340–7344. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAWAY J. L. CHRONIC LEG ULCERS. JAMA. 1963 Dec 21;186:1080–1081. doi: 10.1001/jama.1963.63710120016012. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Sipes N. J., Bascom C. C., Graves-Deal R., Pennington C. Y., Weissman B. E., Moses H. L. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [PubMed] [Google Scholar]
- DUNPHY J. E., UDUPA K. N. Chemical and histochemical sequences in the normal healing of wounds. N Engl J Med. 1955 Nov 17;253(20):847–851. doi: 10.1056/NEJM195511172532002. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., Klagsbrun M., Hill K. E., Buckley A., Sullivan R., Brewer P. S., Woodward S. C. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985 Apr;100(4):1219–1227. doi: 10.1083/jcb.100.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. M., Schiffer S. G., Rohde M. F., Tsai L. B., Banks A. R., Arakawa T. Production, biological activity, and structure of recombinant basic fibroblast growth factor and an analog with cysteine replaced by serine. J Biol Chem. 1988 Dec 5;263(34):18452–18458. [PubMed] [Google Scholar]
- Franklin J. D., Lynch J. B. Effects of topical applications of epidermal growth factor on wound healing. Experimental study on rabbit ears. Plast Reconstr Surg. 1979 Dec;64(6):766–770. doi: 10.1097/00006534-197912000-00003. [DOI] [PubMed] [Google Scholar]
- GRILLO H. C., WATTS G. T., GROSS J. Studies in wound healing: I. Contraction and the wound contents. Ann Surg. 1958 Aug;148(2):145–160. doi: 10.1097/00000658-195808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPH J., TOWNSEND F. J. The healing of defects in immobile skin in rabbits. Br J Surg. 1961 Mar;48:557–564. doi: 10.1002/bjs.18004821121. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W., Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med. 1979 Dec 1;150(6):1421–1431. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Raghow R., Sawdey M., Loskutoff D. J., Postlethwaite A. E., Kang A. H., Moses H. L. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988 Mar 5;263(7):3111–3115. [PubMed] [Google Scholar]
- Krull E. A. Chronic cutaneous ulcerations and impaired healing in human skin. J Am Acad Dermatol. 1985 Feb;12(2 Pt 2):394–401. doi: 10.1016/s0190-9622(85)80002-5. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. W., Peacock E. E., Jr Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery. 1968 Jul;64(1):288–294. [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- O'Keefe E. J., Chiu M. L., Payne R. E., Jr Stimulation of growth of keratinocytes by basic fibroblast growth factor. J Invest Dermatol. 1988 May;90(5):767–769. doi: 10.1111/1523-1747.ep12560956. [DOI] [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Ammann A. J. Suppression of immune cell function in vitro by recombinant human transforming growth factor-beta. Cell Immunol. 1988 Apr 1;112(2):343–350. doi: 10.1016/0008-8749(88)90303-6. [DOI] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Baehner R. L. Platelet-derived growth factor promotes human peripheral monocyte activation. Blood. 1985 Jul;66(1):179–183. [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Senior R. M., Boxer L. A., Baehner R. L. Platelet-derived growth factor promotes polymorphonuclear leukocyte activation. Blood. 1984 Nov;64(5):1123–1128. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]