Abstract
Objective
Atherosclerosis is influenced by the interaction of environmental and genetic susceptibility risk factors. We used global microarray expression profiling to investigate differentially regulated genes in aorta during development of atherosclerosis in a susceptible genetically modified mouse model in response to the interaction between risk factors including hyperlipidemic genotype, shear stress, diet, and age.
Methods and Results
In this study we investigated transcriptional changes in lesion-prone and lesion-resistant regions of aortas in genetically modified mice lacking both genes of the LDL receptor and the apolipoprotein B mRNA editing enzyme (LDb; Ldlr−/−Apobec1−/−). Risk factors including hyperlipidemic genotype (LDb vs. C57BL/6 wildtype), shear stress (lesion-prone vs. lesion resistant aortic regions), diet (chow vs. Western high-fat), and age (2-months vs. 8-months) were studied. We hybridized aortic RNA samples with microarray chips containing probes for 45,000 mouse genes and expressed sequence tags (ESTs). Overall, the differentially expressed genes were components of 20 metabolic and physiological pathways. Notably, calcium signaling is the major pathway identified with differential regulation of 30 genes within this pathway. We also found differential expression of calcium signaling genes in cultured primary endothelial cells from lesion-prone and lesion-resistant arterial regions (LDb mice vs C57BL/6 controls), providing further support for involvement of calcium signaling in the pathogenesis of atherosclerosis. Moreover, we demonstrated protein expression of genes in the calcium signaling pathway using Western blot analysis and immunofluorescence.
Conclusions
Our results suggest that calcium signaling may play an important role in regulation of genes expressed in aorta during development of atherosclerosis. Calcium signaling may act via mechanistic responses to genetic, mechanical, and environmental insults that trigger an imbalance of intracellular calcium homeostasis, resulting in altered biological processes leading to lesion development.
Keywords: atherosclerosis, cDNA microarray, aorta, risk factors, calcium signaling pathway
1. INTRODUCTION
Atherosclerosis is the primary cause of coronary artery disease (CAD) and the leading cause of death in North America. As the genetic details of CAD pathways are being elucidated, it is clear that the development of atherosclerosis involves cascades of molecular interactions between multiple genes and environmental factors, ultimately resulting in the development of pathological lesions. Studies have identified numerous risk factors that contribute to CAD such as hyperlipidemia, aging, diet, smoking, type 2 diabetes, and hypertension. In addition, it is now recognized that shear stress related to blood flow is an independent risk factor in the development of atherosclerosis 1, 2. This concept is based on the observation that atherosclerotic plaques occur preferentially in areas such as the inner curvatures of coronary arteries or near bifurcations, where shear stress is oscillatory 3–5. Although previous studies have investigated changes in gene expression in CAD with regards to shear stress 6, age 7, genetic strain 7, and diet 8, we know of no gene expression study that examined the combined effects of these CAD risk factors on atherogenesis.
We designed this experimental study to investigate the effects of these risk factors on differential gene expression in aorta using a genetic modified mouse model (LDb) that has demonstrated susceptibility to atherosclerosis. Teng and coworkers 9, 10, as well as others 11, developed the LDb mouse model (Ldlr−/−Apobec1−/−) by deleting the genes encoding the LDL receptor (Ldlr) and the apolipoprotein B mRNA editing enzyme (Apobec1). In contrast to wild type mice, the phenotype of the LDb mice closely mimics humans with hyperlipidemia characterized by increased plasma levels of LDL cholesterol (LDL-C) and decreased levels of HDL-C. In addition, these LDb mice spontaneously develop severe atherosclerotic lesions, even when fed on a normal chow diet. Teng and coworkers 9 performed global profiling of hepatic gene expression in LDb mice using microarrays that demonstrated differential expression of genes in pathways for inflammation and calcification. These results were further supported by detection of marked amounts of macrophages and calcification in the aorta of LDb mice 9. In the present study, we analyzed global gene expression of aorta in LDb mice under the influence of four risk factors including hyperlipidemic genotype, diet, aging, and shear stress. We showed that the calcium-signaling pathway is the major controlling factor underlying atherosclerotic lesion development.
2. MATERIALS AND METHODS
2.1 Study Design
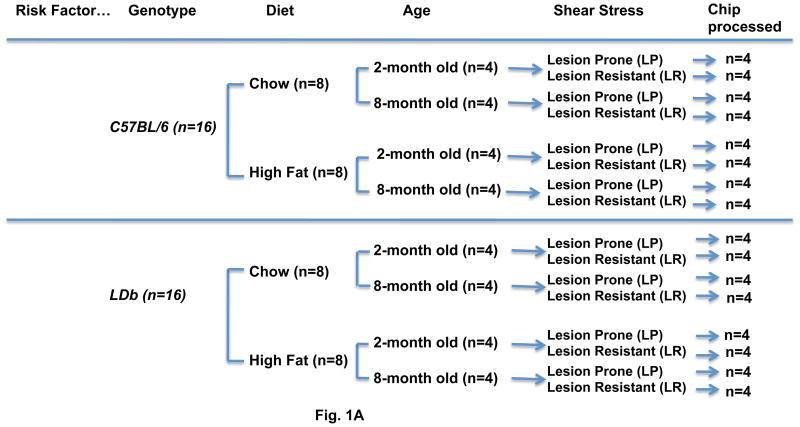
The atherosclerosis susceptible LDb mouse model (Ldlr−/− Apobec1−/− on a C57BL/6 genetic background) was generated in Dr. Teng’s laboratory as previously described 9, 10. Figure 1A shows the experimental design to evaluate aortic differential gene expression influenced by genetic modification (LDb vs. C57BL/6 wild-type), diet (chow vs. Western-high fat diet), age (2-months vs. 8-months), and shear stress (lesion-prone vs. lesion-resistant). Male LDb mice and C57BL/6 control mice (n = 16 per group) were weaned at 4 weeks of age. They were divided and fed with either a chow diet (n=8) or a Western-high fat diet (n=8) (TD 88137, Harlan Teklad, Madison, WI) for a designated time of 1-month or 7-months. From each diet group, four mice were sacrificed at one-month after feeding (2-months-of age) and the other four mice were sacrificed at seven months after feeding (8-months of age). The aorta from each animal was divided into lesion-prone segments of aortic arch (LP) and lesion-resistant segments of the thoracic region (LR) as shown in Fig. 1B for microarray gene expression analysis (Fig. 1A). All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee from the University of Texas Health Science Center at Houston.
Figure 1.
Fig. 1. (A). Flow chart for the study design.
C57BL/6 (n=16) and LDb (n=16) mice of 1-month of age were subjected to either a chow or a Western high-fat diet for one month and 7-months. At the designated time, lesion-prone (LP) and lesion-resistant aortic segments (LR) were collected from each animal for RNA extraction and microarray analysis.
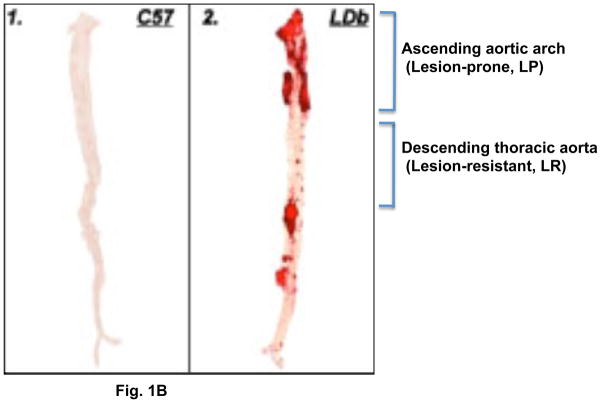
Fig. 1(B). Oil-Red O staining of aorta from C57BL/6 and LDb mice at 8-months of age.
Representative Oil-Red O stained aorta from C57BL/6 (1) and LDb (2) mice are illustrated. The locations of lesion-prone (LP) and lesion-resistant (LR) segments are indicated.
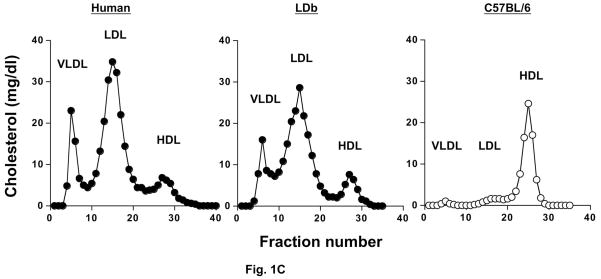
Fig. 1(C). Distribution of cholesterol in plasma separated by FPLC from a male human patient with hyperlipidemia, and for male LDb and C57BL/7 mice.
Pooled plasma samples (200 μl) from 4–6 animals and a patient with hyperlipidemia were fractionated by FPLC. Total cholesterol from each fraction were measured and expressed as mg/dl. The elution fraction for each lipoprotein class is shown.
2.2 RNA preparation, microarray hybridization, and array analysis
The aorta of each mouse was perfused with cold PBS through the left ventricle of the heart. The aorta was cleaned of adventitial fat and connective tissues. The lesion-prone region (LP) was dissected from the ascending aortic root to the fourth rib where the region extended into the thoracic portion. The lesion-resistant region (LR) was the adjacent segment located from LP to the branching of the renal artery. Each segment of the aorta was flash frozen in liquid nitrogen. We extracted total RNA from each tissue segment using Trizol reagent (Invitrogen), followed by Qiagen RNeasy Mini Kit (Qiagen). RNA concentrations were determined by spectrophotometry, and RNA integrity was evaluated on a 1% agarose gel. The yield of total RNA was 3–10 μg from the LP segment and 1–5 μg from the LR segment.
We used 100 ng of total RNA from LP or LR segments to synthesize biotinylated labeled cRNA probe as described in Two-Cycle Eukaryotic Target Labeling for GeneChip Expression Analysis (Affymetrix, Santa Clara, CA). We hybridized 5 μg of fragmented biotinylated cRNA to an Affymetrix Test 3 control chip to assess the probe quality. We then used 15 μg of fragmented cRNA probe to hybridize to an Affymetrix Murine 430, 2.0 microarray chip (Affymetrix Murine 430, 2.0 chip interrogates approximately 45,000 mouse genes and ESTs) at 45°C for 16 hours, followed by wash cycles and stain procedure as described in the EukGE-WS2v5 protocol. The fluorescent hybridization signals were scanned and captured on an Affymetrix Scanner 3000 at Baylor College of Medicine Microarray Core Facility and the fluorescent signal intensities on the array were analyzed using the Gene Chip Operating System (GCOS; Affymetrix) to generate image (.dat) files.
2.3 Microarray analysis
All 64 .dat image files were imported into the dChip software (www.dChip.org) 12 for normalization and analysis. The background artifacts were manually inspected and removed. All the 64 chips were then corrected for local background correction (dChip, ID #6444). After the adjustment, the data was normalized using two methods implemented in the dChip analysis software; a LOWESS algorithm as implemented in the invariant set method (dChip) 13 and a quantile normalization (RMA) method 14. Image intensities above a 20,000 cut-off value were excluded, single and array outliers were regarded as missing values or blank. Probe intensities were extracted and summarized in a Model Based Expression Index (MBEI) file. The average of the median intensities of the 64 chips was 125±45. The average percentage of probes that were detected or present was 48%±11. The average array outlier was 4.8%.
We used t-test for analyzing differential gene expressions. T-test is a robust parametric analysis and is a preferred method when comparing the difference between the mean of two groups with independent variables with normal distribution. The t-statistic in the dChip software was used to compute the difference between groups and its p-value was computed based on the t-distribution, and the degree of freedom was set according to Welch modified two-sample t-test (dChip.org). For all t-test comparisons outlier expression values were treated as missing data in the comparison analysis, we assumed two-tail t-test with equal variance, except for shear stress comparison between lesion-prone and lesion-resistant a paired t-test with equal variance was used instead.
Fold change in gene expression was defined as the average normalized expression signals of mice from the “treatment” group divided by the average normalized expression signal of mice from the “untreated” group (e.g. age=8-months/2-months, diet=high-fat/chow, genotype=LDb/C57, and shear stress=LP/LR). To adjust for multiple comparisons, we randomly permuted the results (100 times each) to estimate the mean empirical false discovery rate (FDR) 15, 16, and chose a FDR of = 0.15 to determine significance of differentially expressed genes. Candidate genes meeting these criteria were imported for pathway analysis.
2.4 Pathway analysis
We submitted our lists of differentially expressed genes and their fold changes from the t-tests for analysis by Pathway Express (http://vortex.cs.wayne.edu). This pathway analysis is free to the public, offers easy and fast data handling, and the pathway networks have been curated. Pathway Express Software used the Kyoto Encyclopedia of Genes and Genomes (KEGG) database 17, 18 to search for functional pathways that are significantly over-represented by differentially expressed genes. The algorithm of the Pathway Express used fold-change of expression for each input gene, positions of genes in pathway hierarchies, and the proportion of genes in the pathway that are differentially regulated to calculate a perturbation factor (PF), which generated the significance of pathways 19, 20. Moreover, PF values were used to derive significance levels to rank the significant over-represented pathways in each risk factor.
2.5 Validation of microarray results by quantitative real-time PCR of calcium signaling gene expression
A set of 17 genes was chosen from 30 differentially expressed genes in the calcium signaling pathway for quantitative real-time PCR (RT-PCR) to validate the differential expression determined by microarray 21, 22. Oligonucleotide primer sets for each gene were designed using Primer Express software (Applied Biosystems). RT-PCR was performed using the SYBR Green master mix reagent (Applied Biosystems) in an ABI Prism 7900HT Sequence Detection System. Briefly, cDNA was produced from each sample using the Achieved cDNA kit (Applied Biosystems). The optimized concentration of each gene-specific primer set was determined as shown in (Supplement Table 1). We used a standard curve method to quantify gene expression, and the relative expression of each gene was normalized with 16S RNA. The PCR reaction was performed using the default protocol: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C. The dissociation curve was obtained using a thermal regime of 15 sec at 95°C, 15 sec at 60°C, and 15 sec at 95°C.
2.6 Isolation and culture of primary vascular endothelial cells
Primary endothelial cells were cultured from lesion-prone and lesion-resistant arterial sections of C57BL/6 and LDb mice at 8-months of age. Briefly, aorta was cleaned as described above. Aortic segments (3 mm in length) were place onto a 35-mm matrigel culture plate containing endothelial media (DMEM media supplemented with 15% FBS, heparin 90 μg/ml, ECGS 60 μg/ml, and 1% Antibiotic-Antimycotic) 23. After approximately 4–7 days of growth, primary endothelial cells were removed from matrigel plates using Dispase, plated onto 0.2% gelatin-coated culture dishes, and expanded in endothelial media.
2.7 Characterization of primary vascular endothelial cells
Uptake of DiI (1,1′-dioetadeeyl-3,3,3′,3′-tetramethylindocarboeyanine perchlorate)-labeled Acetyl-LDL
We plated mouse primary vascular endothelial cells obtained from C57BL/6 and LDb mice as described above and control human EA.hy926 cells (kindly provided by Dr. CoraJean S. Edgell, University of North Carolina, Chapell Hill, NC) in a 0.2% gelatin coated chamber slide overnight. Cells were incubated in media (300 μl) containing 10 μg/ml DiI-Acetyl-LDL (Invitrogen) at 37°C for 6 h. The media was removed; the cells were washed three times with PBS, followed by fixing with 4% buffered formalin phosphate (Fisher Scientific) for 10 min. The slides were mounted with mounting medium containing DAPI (Vector). The image was examined using a Zeiss Axioskop fluorescence microscope (Zeiss Observer.D1m). A Texas Red filter set was used to image DiI.
Immunofluorescence analysis
We cultured human aortic smooth muscle cells (Cambrex), EA.hy926, and primary vascular endothelial cells from C57BL/6 and LDb in a 0.2% gelatin coated chamber slide overnight. The cells were washed with PBS three times, and fixed with 4% buffered formalin phosphate for 10 min. We then incubated the cells with PBS containing 0.25% Triton X-100 to permeabilize the cells, followed by washing with PBS three times. The slides were incubated with 1% BSA in PBST (0.1% Tween-20) for 30 min to block non-specific binding. The cells were then incubated with rabbit anti-CD31 at 1:100 (Fisher Scientific) or rabbit anti-a actin at 1:100 (Abcam) at 4°C overnight. Next day, the cells were washed and incubated with Alexa-594 conjugated secondary goat anti-rabbit IgG at 1:300 for 1h at room temperature. After washing off the secondary antibody, all the slides were examined using a Zeiss Observer.D1m fluorescence microscope with Texas Red and DAPI filters. Control slides without primary or secondary antibodies were performed at the same time.
2.8 RNA extraction and RT-PCR quantification of calcium signaling gene expression in primary vascular endothelial cells
RNA was extracted from lesion-prone and lesion-resistant primary endothelial cells using Trizol (Invitrogen). After treatment with DNase I (Ambion), endothelial cell RNA samples were used for cDNA synthesis with a high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative RT-PCR assays of gene expression for the 17 genes selected from the calcium signaling pathway were performed using the SYBR Green master mix reagent (Applied Biosystems) with the ABI Prism 7900HT Sequence Detection System as described above.
2.9 Western blot analysis
The whole aortas from C57BL/6 and LDb mice at 2- and 8-months of age fed a chow diet were collected as described in Section 2.2 and snap frozen until analysis. The frozen aorta was homogenized in 0.25 ml ice-cold M-PER Mammalian Protein Extraction Reagent (Pierce) containing protease inhibitors (Roche Molecular Biochemicals). After homogenization, tissue homogenate was kept on ice for 30 min, followed by centrifugation at 10,000 × g for 10 min at 4°C. The supernatant was kept at − 80°C until analysis.
Proteins from cultured primary endothelial cells of Lesion-prone (LP) and Lesion-resistant (LR) regions were extracted using the same buffer. Protein concentrations were determined using the Bradford assay (Bio-Rad).
We selected certain genes in the calcium signaling pathway for Western Blot analysis. We used 15 μg protein content to separate proteins by either 6% or 15% SDS-PAGE according to their molecular weight. The proteins were transferred to Immobilon P membranes (Millipore). The membranes were incubated with the following antibodies (Abcam) to detect the presence of each protein: rabbit monoclonal to Calmodulin (ab45689) at 1:1000, mouse monoclonal to SERCA2 ATPase (ab2861) at 1:1000, rabbit polyclonal to GNAQ (ab75825) at 1 μg/ml, rabbit monoclonal to myosin light chain kinase (ab76092) at 1:4000, rabbit polyclonal to prostaglandin E receptor EP3 (ab21227) at 1:1000, and mouse monoclonal to Gapdh (Chemicon International) at 1:3000. The membranes were subsequently incubated with secondary antibody (Invitrogen) at 1:3000 dilution of either Alexa680 conjugated goat-anti-rabbit IgG or Alexa680 conjugated goat -anti-mouse IgG. Each protein was detected and quantified using an Odyssey infrared imaging system (LI-COR). Each protein was normalized with the corresponding Gapdh value. The normalized protein value was used to perform statistical analysis to compare the difference between the groups of aorta-LDb vs. aorta-C57BL/6, or LDb-LP vs. C57BL/6-LP, or LDb-LR vs. C57BL/6-LR. We used unpaired t-test with Welch’s correction implanted in the GraphPad Prism, ver 5.0 (GraphPad Software, Inc., La Jolla, CA), the p value < 0.05 was significant.
2.10 Immunofluorescence analysis on Aortic Sinus
Cryosections (5 micron) of aortic sinus from 8-months of age C57BL/6 and LDb mice fed a chow diet were fixed in 4% buffered formalin phosphate for 10 min at room temperature. After washing, the sections were incubated with 1% BSA PBS-Tween 20 for 1h at room temperature to block nonspecific binding. The sections were then incubated at 4°C overnight with the following primary antibodies, 1:100 dilution of goat anti-CD11b (Pharmingen), 1:100 dilution of goat anti-CD68 (Santa Cruz Biotech), 1:50 dilution of mouse anti-SERCA2 (Abcam), 1:100 dilution of rabbit anti-Calmodulin (Abcam), 1:100 dilution rabbit anti-GNAQ (Abcam), 1:100 dilution rabbit anti-myosin light chain kinase (Abcam), and 1:100 dilution rabbit anti-prostaglandin E receptor EP3 (Abcam). After washing, the slides were incubated with 1:150 Alexa-594 or Alexa-488 conjugated corresponding secondary antibodies (Invitrogen) for 1h at room temperature. The slides were subsequently washed and mounted with mounting medium containing DAPI (Vector) and examined using a Zeiss Axio observer.D1m fluorescence microscopy with DAPI, FITC and Texas Red filters. Control slides without primary or secondary antibodies were performed at the same time.
3. RESULTS
3.1 Metabolic and biochemical characteristics of LDb mice fed chow and high-fat diets
Table 1A shows the plasma parameters for the wild-type C57BL/6 and LDb male mice on regular chow diet or Western high-fat diet. All the mice were weaned at one-month of age and entered the study on a chow or a high-fat diet for the designated time. The body weight of each strain increased steadily from 2 months to 8 months of age, and there were no differences between the two genotypes. The mice on high-fat diet became heavier than the mice on chow diet for both C57BL/6 and LDb mice only at 8-months of age. The levels of plasma cholesterol and triacylglycerol in LDb mice were at least 5 times higher than C57BL/6 mice. As shown by FPLC distribution profiles, similar to hyperlipidemia patients LDb mice had elevated VLDL and LDL cholesterol, whereas most of the plasma cholesterol in C57BL/6 mice was in the HDL fraction (Fig. 1C and Table 1A). In contrast, LDb mice had approximately 20% of plasma cholesterol in the HDL subfraction. Thus, LDb mice have a similar lipoprotein profile as hyperlipidemia patients.
Table 1A. Levels of body weight, plasma cholesterol and triacylglycerol, and ratio of HDL cholesterol to plasma cholesterol in wild-type C57BL/6 and LDb (Ldlr−/−Apobec1−/−) mice after feeding on a chow diet or a Western High-fat diet.
Each value represents the average fasting plasma concentrations of 6–10 mice at the indicated time of diet after one-month of age. Results are presented as means ± S.D. and were analyzed using un-paired student t-test as described in the Materials and Methods section. P values are shown, with p < 0.05 are significant. NS= not significant
| Time on diet (months) | 1 | 4 | 7 | 1 | 4 | 7 | 1 | 4 | 7 | 1 | 4 | 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) | 2 | 5 | 8 | 2 | 5 | 8 | 2 | 5 | 8 | 2 | 5 | 8 |
| Body weight (g) |
TC (mg/dl) |
HDL-C/TC |
TG (mg/dl) |
|||||||||
| Chow | ||||||||||||
| C57 | 26±2.0 | 35±1.7 | 36±2.8 | 82±17 | 81±22 | 84±20 | 1.0±0.12 | 1.41±0.18 | 1.26±0.15 | 51±16 | 45±15 | 38±5.8 |
| LDb | 23±2.5 | 28±3.2 | 37±4.3 | 352±3.1 | 325±56 | 334±78 | 0.17±0.03 | 0.23±0.05 | 0.23±0.03 | 163±37 | 145±36 | 148±63 |
| p | NS | NS | NS | 1.63E-10 | 1.30E-08 | 5.55E-07 | 5.03E-02 | 4.03E-02 | 2.06E-02 | 8.24E-07 | 1.91E-06 | 1.72E-04 |
| High-fat | ||||||||||||
| C57 | 22±1.7 | 34±5.5 | 46±3.3 | 69±8.1 | 215±21 | 333±57 | 1.0±0.14 | 1.1±0.16 | 0.63±0.04 | 25±6.0 | 48±9.5 | 66±11 |
| LDb | 23±1.6 | 33±5.2 | 41±9.2 | 383±95 | 1278±231 | 826±193 | 0.14±0.02 | 0.30±0.02 | 0.25±0.04 | 133±30 | 514±91 | 321±73 |
| p | NS | NS | NS | 1.01E-06 | 5.67E-08 | 4.53E-06 | 4.21E-02 | 2.50E-07 | 4.67E-01 | 2.85E-07 | 2.26E-08 | 2.55E-07 |
There were no obvious effects on plasmid lipid levels after feeding a high-fat diet for one month to either C57BL/6 or LDb mice (Table 1A). However, after 4 months on a high-fat diet, plasma cholesterol levels increased ~3-fold compared to the chow diet in both strains. The plasma triacylglycerol levels did not change much in C57BL/6 mice, but increased markedly (> 3-fold) in LDb mice after feeding a high-fat diet for over 4 months. The high-fat diet decreased the ratio of HDL cholesterol to total cholesterol (HDL-C/TC) in C57BL/6 mice (decreased 20% after 4 months and 50% after 7-months), suggesting that long-term feeding of the high-fat diet increased levels of VLDL and LDL cholesterol and decreased HDL levels in the C57BL/6 mice.
3.2 Atherogenesis in LDb mice
We used the en-face technique to quantify atherosclerotic lesions in the whole aorta to assess lesion development in the mice. The percentage of lesions for the whole aorta in both strains were quantified as shown in Table 1B. As expected, the control C57BL/6 mice on both diets did not develop any lesions throughout the course of the experiment. In LDb mice, there were no detectable lesions at 2-months of age. However, by 8 months of age, > 20% of the aortic surface was covered with lesions on the chow diet. High-fat diet accelerated lesion development in LDb mice, with > 30% of the aortic surface involved in atherosclerotic lesions. As shown in Figure 1B, the LDb mouse had atherosclerotic lesions clustered in the aortic arch and the vessel branching point to the renal arteries, anatomic regions associated with high shear stress. In this study, we used a microarray expression profiling approach to identify genes and pathways that contribute to development of atherosclerotic lesions via differential gene expression in the ascending aortic arch (lesion-prone region) compared to the descending thoracic aorta (lesion-resistant region).
Table 1B. Atherosclerotic lesions in wild-type C57BL/6 and LDb (Ldlr−/−Apobec1−/−) mice after feeding on a chow diet or a Western High-fat diet.
Results are presented as means ± S.D. for the indicated numbers of each genotype. N values are shown in parentheses. Percentage lesions, total atherosclerotic lesion area in mm2/total surface area of the aorta in mm2.
| Time on the diet (months) | 1 | 7 |
|---|---|---|
| Age of the animals | 2 | 8 |
| Chow | ||
| C57BL/6 | 0.54±0.12 (3) | 0.18±0.10 (3) |
| LDb | 0.15±0.10 (3) | 21±6.5 (6) |
| p | ns | 2.2E-05 |
| High-fat | ||
| C57BL/6 | 0.06±0.02 (3) | 0.10±0.10 (3) |
| LDb | 0.17±0.10 (3) | 30±7.2 (6) |
| p | ns | 3.8E-06 |
3.3 Global gene expression analysis
Gene expression in the aorta was compared among four factors including genotype, age, diet, and shear stress. The differentially expressed genes were analyzed using Pathway express software to identify underlying biological pathways. Table 2A shows the top six canonical biological pathways as determined by rank scores. The calcium signaling pathway was ranked the highest with a sum rank score of 0.818, followed by complement and coagulation cascade (0.682), focal adhesion (0.676), phosphatidyl-Inositol signaling system (0.643), cytokine-cytokine receptor interaction (0.530), and MAPK signaling pathway (0.410).
Table 2A. Canonical pathway analysis of genes differentially expressed in aorta analyzed from the comparison of genotype, age, diet, and sheer stress.
The differentially expressed genes were analyzed using Pathway express software to identify underlying biological pathways as described in the Materials and Methods section.
ns= not significantly
| Over-represented KEGG pathways | Sum of rank scores | Number of genes expressed in the pathway | P-value | |||
|---|---|---|---|---|---|---|
| Genotype | Age | Diet | Shear Stress | |||
| Calcium Signaling Pathway | 0.818 | 30 | 0.0021 | ns | 0.0002 | 0.00064 |
| Complement and Coagulation cascades | 0.682 | 17 | 0.017 | 0.00021 | 0.0011 | 0.02 |
| Focal adhesion | 0.676 | 26 | 0.0028 | 0.0063 | 0.074 | ns |
| Phosphatidyl-Inositol signaling system | 0.643 | 12 | 0.022 | 0.011 | ns | 0.00083 |
| Cytokine-cytokine receptor interaction | 0.530 | 44 | ns | 3.1E-13 | 0.0013 | ns |
| MAPK signaling pathway | 0.410 | 19 | 0.031 | ns | 0.036 | 0.025 |
Next, we analyzed the biological pathways of differentially expressed genes for each individual factor. As shown in Table 2A, there were 30 genes that were differentially regulated in the calcium signaling pathway, with highly significant p values for the pathway for genotype (p=0.0021), diet (p=0.0002), and shear stress (p=0.00064). Age did not play a significant role in the pathway of calcium signaling. There were 17 differentially expressed genes in the complement and coagulation cascade, with significant values for all four risk factors. The focal adhesion pathway had 26 differentially expressed genes, with significant p-values for genotype, age, and diet, but not shear stress. The phosphatidyl-inositol signaling pathway had 12 differentially expressed genes, and was not significant for the diet factor. The cytokine-cytokine receptor interaction pathway had 44 genes represented, with significant p values for age and diet. The MAPK signaling pathway had 19 differentially expressed genes with significant p-values for genotype, diet, and shear stress.
In Table 2B, we list the fold changes for differential expression of 30 unique genes in the calcium signaling pathway for the corresponding risk factors. In the calcium-signaling pathway, genotype was the risk factor that affected the most number of genes, followed by shear stress, age, and diet.
Table 2B. The fold-changes of differentially expressed genes in the calcium signaling pathways from comparison of genotype, diet, age, and shear stress.
The value of fold-change in each comparison was obtained by taking the average normalized expression signal in the treatment group divided by the average expression signal in the control group. The ↑ represents up-regulation and ↓ represents down-regulation. The p values were obtained as described in the Methods
| Gene Symbol | Gene Name | Affymetrix ID | Comparison | Fold-changes | P values |
|---|---|---|---|---|---|
| Calcium signaling pathway (30 unique genes) | |||||
| Adora2b | Adenosin A2b receptor | 1434431_x_at | Age | ↑1.78 | 1.30E-03 |
| Atp2a2 (SERCA) | ATPase, Calcium transporting, cardiac muscle, slow twitch 2channel | 1416551_at | Stress | ↑2.46 | 4.00E-04 |
| Diet | ↓2.35 | 5.20E-03 | |||
| Atp2b1 | ATPase, Calcium transporting, plasma membrane 1 | 1428937_at | Genotype | ↑1.83 | 3.30E-03 |
| Atp2b2 | ATPase, Calcium transporting, plasma membrane 2 | 1420402_at | Stress | ↓1.21 | 1.27E-02 |
| Cacna1c | Calcium channel, voltage dependent, L type, alpha 1C subunit | 1421297_a_at | Stress | ↑1.73 | 7.00E-04 |
| Cacna1d | Calcium channel, voltage dependent, L type, alpha 1D subunit | 1427974_s_at | Genotype | ↓1.79 | 2.25E-02 |
| Camk4 | Calcium/calmodulin-dependent protein kinasee | 1438960_at | Diet | ↑1.55 | 4.30E-03 |
| Cd38 | CD38 antigen | 1433741_at | Genotype | ↑5.35 | 1.08E-04 |
| Age | ↑1.58 | 1.00E-04 | |||
| Cdc2a | Cell division cycle 2 homolog A | 1448314_at | Age | ↑1.56 | 1.00E-03 |
| Gnaq | Guanine nucleotide binding protein | 1447593_x_at | Genotype | ↓1.52 | 3.53E-02 |
| Age | ↑1.56 | 7.60E-03 | |||
| Htr2b | 5-hydroxytrytamine (serotonin receptor 2B) | 1422125_at | Genotype | ↑1.64 | 1.50E-03 |
| Age | ↑2.39 | 1.50E-03 | |||
| Htr2c | 5-hydroxytrytamine (serotonin receptor 2C) | 1435513_at | Genotype | ↓1.62 | 2.85E-02 |
| Mlck (Mylk) | myosin, light polypeptide kinase | 1439101_at | Stress | ↑3.86 | 2.00E-04 |
| Diet | ↓4.20 | 2.80E-03 | |||
| Genotype | ↑8.34 | 8.00E-04 | |||
| P2rx1 | purinergic receptor P2X, ligand-gated ion channel, 1 | 1460719_a_at | Stress | ↓1.49 | 4.60E-03 |
| Plcb4 | phospholipase C, beta 4 | 1441531_at | Genotype | ↓6.74 | 5.00E-04 |
| Pln | phospholamban | 1450952_at | Stress | ↑6.59 | 7.00E-05 |
| Age | ↑8.46 | 6.78E-03 | |||
| Ppp1r12a | protein phosphatase 1, regulatory (inhibitor) subunit 12A | 1444762_at | Genotype | ↓3.48 | 1.00E-05 |
| Ppp3ca | protein phosphatase 3, catalytic subunit, alpha isoform | 1426401_at | Genotype | ↑1.75 | 3.30E-03 |
| Ppp3cb | protein phosphatase 3, catalytic subunit, beta isoform | 1459378_at | Diet | ↑1.50 | 4.37E-02 |
| Prkcb1 | protein kinase C, beta | 1460419_a_at | Age | ↑1.96 | 1.00E-03 |
| Ptger1 | prostaglandin E receptor 1 (subtype EP1) | 1445445_s_at | Stress | ↓1.24 | 4.84E-02 |
| Ptger3 (EP3) | Prostagladin E receptor 3 (subtype EP3) | 1450344_a_at | Stress | ↓2.45 | 1.00E-05 |
| Ptger4 | prostaglandin E receptor 4 (subtype EP4) | 1421073_a_at | Stress | ↑1.50 | 2.40E-03 |
| Ptgfr | Prostagladin F receptor | 1420349_at | Stress | ↑1.25 | 2.54E-02 |
| Diet | ↓2.48 | 1.40E-03 | |||
| Pygl | liver glycogen phosphorylase | 1417741_at | Stress | ↓1.84 | 1.50E-03 |
| Ryr2 | Ryanodine receptor 2, cardiac | 1450123_at | Diet | ↓6.23 | 9.33E-03 |
| Slc25a4 | solute carrier family 25 (mitochondrial carrier, adenine nucleotide translocator), member4 | 1455069_x_at | Genotype | ↑2.45 | 9.70E-03 |
| Slc8a1 | solute carrier family 8 (sodium/calcium exchanger), member 1 | 1445481_at | Genotype | ↓2.85 | 1.00E-04 |
| Syk | spleen tyrosine kinase | 1418261_at | Age | ↑1.95 | 4.80E-03 |
| Vdac1 | Voltage-dependent anion channel 1 | 1415998_at | Genotype | ↑2.75 | 1.85E-04 |
| Diet | ↓2.58 | 1.40E-03 | |||
3.4 Quantitative RT-PCR confirmation of microarray results for differentially expressed genes in the calcium signaling pathway
The calcium-signaling pathway was ranked as the most significant pathway in this study with 30 genes that were differentially expressed. We used quantitative RT-PCR to confirm the results of microarray assays for 17 of the differentially expressed genes. The fold changes calculated from RT-PCR assays correlated well with microarray assays (correlation coefficient was 0.82).
3.5 Perturbation of the calcium signaling pathway in endothelium during atherogenesis
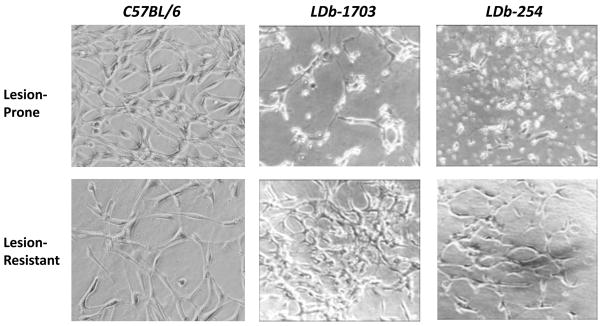
To determine whether the calcium signaling pathway plays an important role in vascular endothelial cells, we isolated and cultured primary vascular endothelial cells from lesion-prone and lesion-resistant sections of LDb and C57BL/6 mice. In here, we focused on the 2 factors, genotype and shear stress. As shown in Figure 2, primary endothelial cells from both lesion-prone and lesion-resistant sections of C57BL/6 mice formed tubular structures. Unlike C57BL/6 mice, cells from lesion-prone sections of LDb mice (mouse # 1703 and 254) were disorganized, whereas cells from lesion-resistant sections formed tubular structures. Thus, the endothelial cells were morphologically altered by the development of atherosclerosis.
Fig. 2. Morphology of cultured primary endothelial cells of lesion-prone and lesion-resistant aortic segments from LDb mice.
Endothelial cells were cultured from lesion-prone (LP) and lesion-resistant (LR) aortic segments from 2 LDb mice (#1703 and 254). The aortic segments were placed on Matrigel gel until day 7. The morphology of endothelial cells was captured by phase contrast photomicrographs at 10× magnification.
To characterize these endothelial cells, we incubated the primary cultured endothelial cells obtained from LDb mice and C57BL/6 mice, as well as the control EA.hy926 endothelial cells with DiI-AcLDL (Supplement Fig. 1A). All of these cells took up acetylated LDL. We hybridized the cells with anti-CD31 (Supplement Fig. 1B), the EA.hy926 endothelial cells and endothelial cells from LDb and C57BL/6 mice stained positive with CD31, whereas aortic smooth muscle cells (AoSMC) stained negative with CD31. We also stained these cells with anti-α-actin (Supplement Fig. 1B), as expected, only AoSMCs were detected positive immunofluorescence signal with α-actin, not endothelial cells. Thus, we confirmed that these primary cultured cells are endothelial cells.
Next, we used RT-PCR to quantify transcripts from the 17 selected genes in the calcium signaling pathway (Table 3) in these endothelial cells. The LP/LR gene expression ratios were altered in primary vascular endothelial cells in both C57BL/6 mice and LDb mice, and during atherogenesis. For example, gene expression of Ptger3 decreased 3.64-fold in primary endothelial cells (Table 3), corresponding to a decrease from the microarray results (↓2.45-fold) (Table 2B). Slc8a1 gene expression decreased 1.65-fold, corresponding to a decrease in the microarray results (↓2.85-fold) (Table 2B). Vdac1 gene expression increased 2.86-fold, corresponding to an increase in the microarray results (↑2.75-fold) (Table 2B). We found good correspondence of differential gene expression in the primary endothelial cells with microarray results for Mlck (↑3.71-fold), Plcb4 (↓1.47-fold), and Pln (↑2.86-fold). However, primary endothelial cell expression did not correlate well with microarray results for Ptgfr (↓1.98-fold vs. ↑1.25-fold), Gnaq (↑1.79-fold vs. ↓1.52-fold), and Atp2a2 (no change vs. ↑2.46-fold). Taken together, these findings of differential gene expression in arterial segments (microarray) and primary vascular endothelial cells (RT-PCR) provide strong evidence for marked perturbation of calcium-signaling in endothelium during atherogenesis.
Table 3. Differentially expressed genes in calcium signaling pathway in primary endothelium from lesion prone and lesion resistant aorta of C57BL/6 and LDb mice.
The value of LP/LR from C57BL/6 mice represents average gene expression of LP=3 divided of LR=3 from each gene. The value of LP/LR from LDb mice represents average gene expression of LP=6 divided of LR=7 from each gene LP = lesion prone; LR = lesion resistant; ↑ = up-regulation; ↓ = down-regulation
| Gene Symbol | Gene Name | C57BL/6 | LDb | During Atherosclerosis | Location |
|---|---|---|---|---|---|
| Receptor: | LP/LR | LP/LR | LDb/C57BL/6 | ||
| Egfr | Epidermal growth factor receptor | 2.26 | 0.80 | ↓2.83 | Plasma membrane |
| Htr2b | 5-hydroxytrytamine (serotonin receptor 2b) | 2.06 | 2.01 | no change | Plasma membrane/cytoplasma |
| Ptger3 | Prostagladin E receptor 3 | 1.60 | 0.44 | ↓3.64 | Plasma membrane |
| Ptgfr | Prostagladin F receptor | 3.03 | 1.53 | ↓1.98 | Plasma membrane |
| Ryr2 | Ryanodine receptor 2 (component of a calcium channel) | 2.06 | 1.14 | ↓1.81 | Plasma membrane/Nucleus |
| Tbxa2r | Thromboxane A2 receptor | 0.58 | 2.23 | ↑3.85 | |
| Calcium Channel: | |||||
| Atp2a2 (SERCA) | Atpase, Calcium transporting channel | 1.61 | 1.66 | no change | Endoplasmic reticulum (ER) |
| Cacna1c | Calcium channel, voltage dependent, L type, alpha1 c subunit | 8.06 | 1.52 | ↓5.30 | Plasma membrane |
| Slc8a1 | Solute carrier protein | 1.91 | 1.16 | ↓1.65 | mitochondria |
| Vdac1 | Voltage-dependent anion channel 1 | 0.56 | 1.60 | ↑2.86 | mitochondria |
| G-protein: | |||||
| Gnaq | Guanine nucleotide binding protein | 0.80 | 1.43 | ↑1.79 | Plasma membrane |
| Kinase protein: | |||||
| Calm1 | Calmodulin 1, phosphorylase kinase | 0.84 | 1.68 | ↑2.0 | Cytoplasm/nucleus |
| Camk2d | Calcium/calmodulin-dependent protein kinasee | 0.85 | 1.39 | ↑1.64 | Cytoplasm/nucleus |
| Ip3r1 | Inositol 1,4,5-trisphosphate receptor, type 1 | 1.57 | 0.71 | ↓2.21 | ER |
| Mlck (Mylk) | myosin light chain kinase | 0.58 | 2.15 | ↑3.71 | Cytoplasm |
| Plcb4 | phospholipase C, beta 4 | 1.43 | 0.97 | ↓1.47 | Cytoplasm |
| Pln | phospholamban | 0.56 | 1.60 | ↑2.86 | Plasma membrane |
3.6. Western blot analysis of protein expression
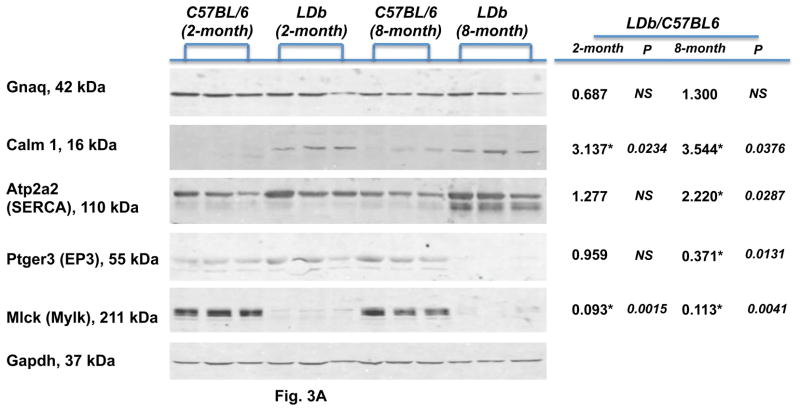
To determine whether the observed differences in gene expression were accompanied by changes in protein abundance, we performed Western blot analysis to estimate the levels of protein expression of five genes (Gnaq, Calm1, Atp2a2, Ptger3, and Mlck) in the calcium signaling pathway. We compared the protein expression of the whole aorta between control C57BL/6 and LDb mice fed on a chow diet at 2- and 8-months of age (Fig. 3A). There was no significant difference in protein levels of Gnaq between control C57BL/6 and LDb mice at 2- or 8-months old. The protein levels of Calm1 (calmodulin) in LDb mice increased significantly at both 2-months and 8-months of age, when compared to C57BL/6 mice (p=0.0234 and 0.0376, respectively). There was no significant difference in the protein levels of Atp2a2 (SERCA) at 2-months of age between LDb and C57BL/6 mice. However, at 8-months of age the protein levels of Atp2a2 (SERCA) in LDb mice was significantly increased (p=0.0287) in comparison to that in C57BL/6. The protein levels of Ptger3 (EP3) decreased substantially in LDb mice at 8-months of age (↓3.3-fold, p=0.0131). In general, the protein expression levels corroborated relatively well with the fold changes in gene expressions as shown in Table 2B; Gnaq decreased 1.52-fold in genotype, Atp2a2 increased 2.46-fold in stress factor, and Ptger3 decreased 2.45-fold in stress factor. The exception is the Mlck gene. The aortic protein expressions of Mlck in LDb mice at both 2-months and 8-months of age decreased markedly (~10-fold, p=0.0015, 0.0041, respectively) in comparison with that in C57BL/6 mice. This finding was opposite to the fold-changes of gene expression of Mlck; the gene expression levels increased 8.34-fold in genotype and 3.86-fold in stress factor (Table 2B).
Figure 3.
Fig. 3A. Western blot analysis of genes associated with calcium signaling pathway in aortic tissues obtained from C57BL/6 and LDb mice
Selected proteins associated with calcium signaling pathway were analyzed by Western blot in aortic tissues obtained from C57BL/6 and LDb mice at 2-months and 8-months of ages fed a chow diet. Aortic homogenates (15 μg) were separated by SDS/PAGE. Protein abundances of Gnaq, Calm1, Atp2a2 (SERCA), Ptger3 (EP3), and Mlck (Mylk) were analyzed and quantified using an Odyssey infrared imaging system (LI-COR). Gapdh was used as control to demonstrate equal sample loading to each lane. Each protein was normalized with the corresponding Gapdh value. Statistical analysis was performed as described in the Methods. The symbol of * represents significant difference of p< 0.05. The ratios of LDb/C57BL6 of 2-month and 8-month of each protein are shown.
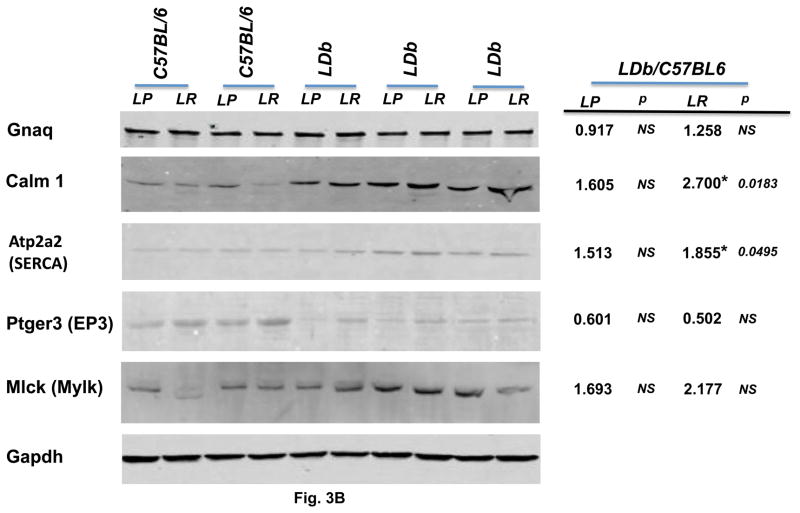
Fig. 3B. Western blot analysis of genes associated with calcium signaling pathway in primary aortic endothelial cells obtained from C57BL/6 and LDb mice
Aortic endothelial cell protein (15 μg) of LP and LR from C57BL/6 and LDb mice were separated by SDS/PAGE. Protein abundances of Gnaq, Calm1, Atp2a2 (SERCA), Ptger3 (EP3), and Mlck (Mylk) were analyzed and quantified using an Odyssey infrared imaging system (LI-COR). Gapdh was used as control to demonstrate equal sample loading to each lane. . Each protein was normalized with the corresponding Gapdh value. Statistical analysis was performed as described in the Methods. The symbol of * represents significant difference of p< 0.05. The ratios of LDb/C57BL6 of LP and LR of each protein are shown.
The protein expression levels of primary LP and LR endothelial cells from C57BL/6 and LDb mice were shown in Fig. 3B. In here, we compared the protein expression levels of LDb to C57BL/6 (LDb/C57BL6). The protein expression levels of LP and LR from LDb endothelial cells were increased in Gnaq, Calm1, Atp2a2, and Mlck genes in comparison with that of C57BL/6. These results correlated relatively well with gene expression levels presented at Table 3 column of “during atherosclerosis” (↑1.79, ↑2.0, no change, and ↑3.71, respectively). However, only the difference of Calm1-LR and Atp2a2-LR protein between LDb and C57BL/6 derived cells reached significant levels (p=0.0183 and p=0.0495, respectively). The Ptger3 protein expressions decreased markedly in LDb, compared to C57BL/6, which was in the same direction as gene expression levels (↓3.64, Table 3). Taken together, the differential changes of endothelial cells protein expressions of Gnaq, Calm1, Atp2a2, Ptger3, and Mlck between C57BL and LDb were similar to that in gene expressions of endothelial cells. Furthermore, except for the Mlck protein, the differential changes in endothelial cells proteins were in the same direction as proteins detected in the whole aorta.
3.7. Detection of protein expression in aorta
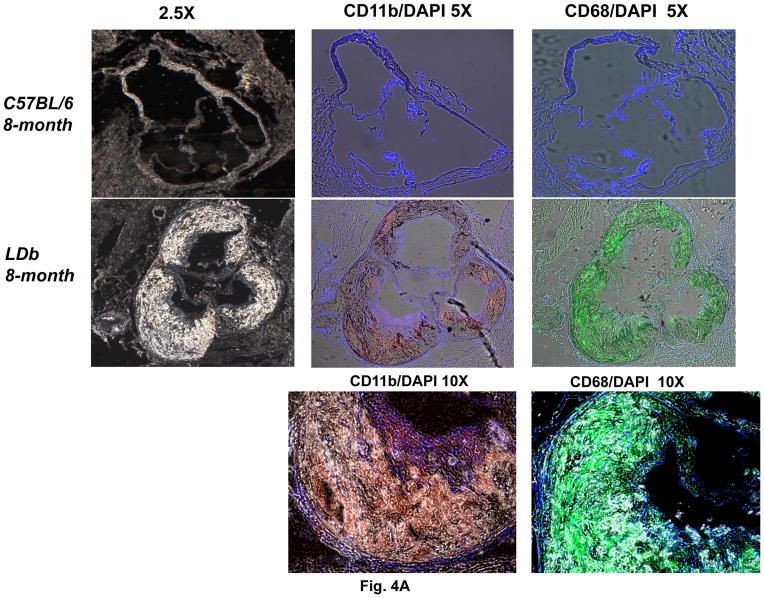
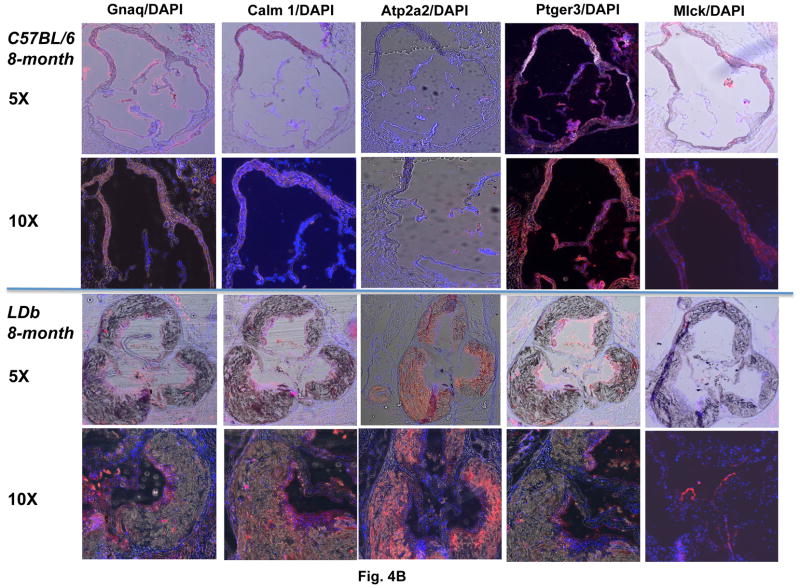
The differences in Mlck protein expression between whole aorta and endothelial cells led us to examine the localization of Mlck protein in aorta using immunofluorescence techniques. We performed this study on aortic sinus of C57BL/6 and LDb mice fed a chow diet at 8-months of age. As shown in Fig. 4A, the aortic sinus of LDb mouse contained extensive atherosclerotic lesions, whereas the C57BL/6 mouse was normal. The lesions contained an abundance of monocytes/macrophages that stained strongly with CD11b and CD68 antibodies. Fig. 4B illustrates the protein expression of Gnaq, Calm1, Atp2a2 (SERCA), Ptger3 (EP3), and Mlck in aortic sinus. As shown, Gnaq, Calm1, and Ptger3 proteins were detected mainly on the luminal layer of the aorta that contains endothelial cells and smooth muscle cells, but were not present within the atherosclerotic lesions. In contrast, Atp2a2 (SERCA) was detected mainly within the atherosclerotic lesions. Furthermore, Mlck expressed vividly in the aorta of C57BL/6 mouse, but was barely detectable in the LDb mouse. This result corresponded well with the protein expression of aorta by Western blot analysis. Taken together, the immunofluorescence studies show that Gnaq, Calm1, and Ptger3 proteins are expressed mainly in the outer luminal layer of the aorta, and Atp2a2 (SERCA) protein was expressed mainly within the atherosclerotic lesions.
Figure 4.
Fig. 4A. The expression of CD11b and CD68 in atherosclerotic lesions in LDb mice
Immunofluorescence staining of aortic sinus sections isolated from C57BL/6 and LDb mice of 8-months of age fed a chow diet were immunostained with anti-CD11b or anti-CD68. The images were captured using a Zeiss Axio observer.D1m fluorescence microscopy with DAPI, FITC and Texas Red filters.
Fig. 4B. The expression of Gnaq, Calm1, Atp2a2, Ptger3, and Mlck in atherosclerotic lesions in LDb mice
Immunofluorescence staining of aortic sinus sections isolated from C57BL/6 and LDb mice of 8-months of age fed a chow diet were immunostained with anti-Gnaq, anti-Calm1, anti-Atp2a2, anti-Ptger3, or anti-Mlck. The images were captured using a Zeiss Axio observer.D1m fluorescence microscopy with DAPI and Texas Red filters.
4. DISCUSSION
We have previously studied hepatic global gene expression of LDb mice (Ldlr−/−Apobec1−/−) 9. That study revealed differential-expression of calcium signaling genes in the liver influenced by high fat diet. In this study, we investigated genes and pathways in the aorta that contribute to atherosclerotic lesion development in LDb mice. We compared gene expression in aortic wall of four risk factors including genotype (LDb vs. C57BL/6 wild-type), age (2-months vs. 8-months), diet (chow vs. Western high-fat), and shear stress (lesion-prone versus lesion-resistant regions). We discovered six biological pathways that were over-represented for differentially expressed genes including calcium signaling, complement and coagulation cascades, focal adhesion, phosphatidylinositol signaling, cytokine-cytokine receptor interaction and MAPK signaling (Table 2A). Among these pathways, the calcium signaling pathway was the most over-represented, with differential gene expression for 30 out of 185 curated calcium-signaling genes in the KEGG pathway (http://www.genome.jp/kegg/pathway.html) (Supplement Figure 2 and Table 2B).
Calcium signaling plays a vital role in a wide ranges of metabolic and physiological processes 24–26. Concentrations of intracellular calcium must be tightly regulated to maintain a proper balance for cellular function. Elevation or prolonged increases in intracellular concentrations of calcium can lead to irreversible cell death due to impaired mitochondrial function and release of cytochrome C that activates phospholipases, proteases, and endonucleases 26. In addition, high intracellular concentrations of calcium are associated with diseases that involve abnormal regulation of cellular processes including inflammation, cell growth and proliferation, thrombosis, oxidative stress, and calcification. Likewise, low intracellular concentrations of calcium are also destructive, leading to activation of stress signaling pathways that activate other genes involved with apoptosis. Many of these cellular processes are involved in development of atherosclerosis, and have been associated with alterations in calcium signaling in the vascular wall. Recent studies have implicated alterations in calcium signaling in macrophage apoptosis induced by oxidized LDL, a process important in formation of necrotic cores that may contribute to vulnerability to plaque rupture 27, 28. A recent study by Yuan and coworkers 29 that compared inbred mouse strains C57BL/6 and C3H/HeJ revealed that the calcium signaling pathway controls atherosclerosis susceptibility.
Extracellular stimuli initiate entry of calcium ion via activated channels and receptors located on the plasma membrane, and by release of calcium from the intracellular storage compartments (ER/SR and mitochondria) to the cytoplasm to modulate cellular processes. During atherogenesis, gene expression of plasma membrane receptors (Egfr, Ptger3, Ptgfr, and Ryr2) were all down regulated in primary endothelial cells, as well as the calcium channel Cacna1c (Table 3). The protein levels of Ptger3 (EP3) were decreased in LDb aorta (Fig. 3A) and endothelial cells (Fig. 3B). Moreover, gene expression levels (Table 2B and 3) of both Ip3r1 (regulates the release of Ca++ from ER to cytoplasm) and PLcb4 (regulates Ip3r1 activity) were also decreased. These results suggest that both Ca++ entry and Ca++ release that introduce Ca++ into the cytoplasm were down regulated.
To achieve low cellular calcium concentration, cells need to sequester cytosolic calcium ions in the mitochondria and ER/SR storage compartments. To sequester Ca++ in the ER and mitochondria, the Pln gene was up regulated to control the activity of Atp2a2 (Tables 2B and 3). The aortic Atp2a2 (SERCA2) protein increased markedly in LDb mice (Fig. 3A and 3B) and it was detected in atherosclerotic lesions (Fig. 4B), which suggests that during atherogenesis Atp2a2 is actively pumping the Ca++ from cytoplasm back to ER to modulate cell injury. SERCA2 has been shown to alter Ca++ release in Apoe−/− mice before development of atherosclerotic lesions 30. The levels of SERCA2 increased in dyslipidemic pigs 31 and in cells treated with oxidized LDL 32. Thus, SERCA2 plays an important role in maintaining Ca++ homeostasis, and atherosclerotic lesion development. Other genes were also involved in maintaining Ca++ levels; cells can directly pump calcium ions into the mitochondria via the voltage-dependent anion channel 1 (Vdac1), which is up-regulated during atherogenesis (Table 3). Cells can inhibit release of calcium ions from mitochondria to the cytoplasm via a family of solute carrier proteins such as Slc8a1, which is down-regulated during atherogenesis (Table 3). In addition, Slc25a4 gene expression was up-regulated in LDb mice, probably to bring Ca++ from the cytoplasm to the mitochondria (Table 2B).
To compensate for depletion of Ca++ in the cytoplasm, Gnaq is expressed in a constant levels (Fig. 3A and 3B) to maintain Ca++ concentration in the cytoplasm. In turn, activation of Calm1 and downstream genes such as Mlck and Camk to regulate muscle contraction, cell proliferation, and cell apoptosis. Thus, large numbers of genes in the calcium signaling pathway are dynamically regulated in atherogenesis to modulate cell injury and survival. In human, Mlck (Mylk) has been shown to be associated with early onset coronary artery disease 33, 34. Mlck plays an essential role in regulating the influx of Ca++ across the plasma membrane in endothelial cells, which regulates endothelial cell retraction 35. In our study, the discordance between gene expression and protein expression levels of Mlck suggested that Mlck protein might be degraded very easily, or it is possible that a factor could inhibit the protein expression of Mlck. The markedly decreased of Mlck protein also suggested the decreased control of contractile capacity of endothelial cells. A further study in this direction is warranted.
In our previous study, we show that hepatic calcium signaling in LDb mice was perturbed after high fat diet treatment 9. The hepatic genes associated with calcium signaling were not the same as the aortic genes of this study. These two studies were designed very differently; the hepatic study compared LDb mice only, whereas the current study compared C57BL/6 control mice to LDb mice. However, both studies suggested disarray of the calcium signaling pathway plays an important role in atherosclerotic lesions development. Recinos et al. 36 examined liver gene expression associated with diet and lesion development in Ldlr−/− mice. They suggested that the complement pathway was perturbed. Complement cascade was the 2nd pathway identified in our study. A recent work by Collins et al. 37 demonstrated that aging accelerated atherosclerosis as the result of increased gene expressions associated with oxidative stress in Ldlr−/− mice. Taken together, the diversity of genes associated with atherosclerosis in different animal models indicates the complexity of atherosclerosis development.
Our study of global gene expression in aorta using the atherosclerosis susceptible LDb mouse model identified the calcium-signaling pathway as the most significant pathway that contributes to atherosclerotic lesion development. This study suggested that risk factors of shear stress, genotype (hyperlipidemia), and diet (high-fat), except age influenced gene expression that may alter calcium signaling resulting in progression of lesion development. The next step will be experiments to manipulate expression of genes in the calcium-signaling pathway to investigate their effects on prevention of atherosclerosis.
5. Conclusion
Using a mouse model lacking both Ldlr and Apobec1 genes, we identified 30 out of 185 calcium signaling genes in the KEGG database that were significantly and differentially expressed in this mouse model. Of particular interest is how these risk factors alter the expression plasma membrane receptors and channels that control intracellular cellular calcium flux and signaling, and how are these changes impact cellular and biological processes resulting in pathological development of atherosclerosis. Whether calcium pathways are primarily responsible for the increase in atherosclerosis susceptibility remains to be elucidated. This work suggests further investigation of the biological role of calcium signaling genes in the initiation and progression of cardiovascular disease.
Supplementary Material
Human endothelial cells (EA.hy926) and mouse primary aortic endothelial cells obtained from C57BL/6 and LDb mice were plated onto chamber slides overnight. The cells were incubated with DiI-Acetyl LDL (10 μg/ml) for 6 h, and mounted with mounting media containing DAPI. The images were examined using Texas Red and DAPI filter sets at 10X magnification. DiI-Acetyl-LDL was taken up by EA.hy926 cells and primary aortic endothelial cells from C57BL/6 and LDb mice.
Human aortic smooth muscle cells (AoSMC), EA.hy926 cells, and primary aortic endothelial cells obtained from C57BL/6 and LDb mice were plated onto chamber slides overnight. The cells were hybridized with anti-CD31 at 1:100 (left panel) or anti-α-Actin at 1:100 (right panel), followed by detection using Alexa-594 conjugated secondary goat anti-rabbit IgG. The images were examined using Zeiss Observer.D1m fluorescence microscope with Texas Red and DAPI filter sets at 10× and 40× magnification. EA.hy926 cells and primary aortic endothelial cells from C57BL/6 and LDb mice stained positive for CD31, whereas AoSMC were negative for CD31. And, only AoSMC stained positive for -α-Actin, but not EA.hy926 or primary aortic endothelial cells of C57BL/6 and LDb.
Pathway Express was used to calculate and curate 30 genes that were over-represented in the calcium signaling pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG). The up-regulated genes are shown in red and the down-regulated genes are shown in blue. The green color is the default color of the pathway.
Acknowledgments
This work was supported by a grant from the NIH (HL084594). Ms. Frances Acevado is a recipient of Minority Access to Research Careers.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 2.Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 3.Schwenke DC, St Clair RW. Accumulation of 125I-tyramine cellobiose-labeled low density lipoprotein is greater in the atherosclerosis-susceptible region of White Carneau pigeon aorta and further enhanced once atherosclerotic lesions develop. Arterioscler Thromb. 1992;12:446–460. doi: 10.1161/01.atv.12.4.446. [DOI] [PubMed] [Google Scholar]
- 4.Stehbens WE. Chronic changes in the walls of experimentally produced aneurysms in sheep. Surg Gynecol Obstet. 1979;149:43–48. [PubMed] [Google Scholar]
- 5.Wagner JD, St Clair RW, Schwenke DC, Shively CA, Adams MR, Clarkson TB. Regional differences in arterial low density lipoprotein metabolism in surgically postmenopausal cynomolgus monkeys. Effects of estrogen and progesterone replacement therapy. Arterioscler Thromb. 1992;12:717–726. doi: 10.1161/01.atv.12.6.717. [DOI] [PubMed] [Google Scholar]
- 6.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–653. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 7.Tabibiazar R, Wagner RA, Spin JM, Ashley EA, Narasimhan B, Rubin EM, Efron B, Tsao PS, Tibshirani R, Quertermous T. Mouse strain-specific differences in vascular wall gene expression and their relationship to vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:302–308. doi: 10.1161/01.ATV.0000151372.86863.a5. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Gargalovic P, Wong J, Gu JL, Wu X, Qi H, Wen P, Xi L, Tan B, Gogliotti R, Castellani LW, Chatterjee A, Lusis AJ. Hyplip2, a new gene for combined hyperlipidemia and increased atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1928–1934. doi: 10.1161/01.ATV.0000143385.30354.bb. [DOI] [PubMed] [Google Scholar]
- 9.Dutta R, Singh U, Li TB, Fornage M, Teng BB. Hepatic gene expression profiling reveals perturbed calcium signaling in a mouse model lacking both LDL receptor and Apobec1 genes. Atherosclerosis. 2003;169:51–62. doi: 10.1016/s0021-9150(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 10.Singh U, Zhong S, Xiong M, Li TB, Sniderman A, Teng BB. Increased plasma non-esterified fatty acids and platelet-activating factor acetylhydrolase are associated with susceptibility to atherosclerosis in mice. Clin Sci (Lond) 2004;106:421–432. doi: 10.1042/CS20030375. [DOI] [PubMed] [Google Scholar]
- 11.Powell-Braxton L, Veniant M, Latvala RD, Hirano KI, Won WB, Ross J, Dybdal N, Zlot CH, Young SG, Davidson NO. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat Med. 1998;4:934–938. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MP, Grundy WN, Lin D, Cristianini N, Sugnet CW, Furey TS, Ares M, Jr, Haussler D. Knowledge-based analysis of microarray gene expression data by using support vector machines. Proc Natl Acad Sci U S A. 2000;97:262–267. doi: 10.1073/pnas.97.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irizarry RA, Warren D, Spencer F, Kim IF, Biswal S, Frank BC, Gabrielson E, Garcia JG, Geoghegan J, Germino G, Griffin C, Hilmer SC, Hoffman E, Jedlicka AE, Kawasaki E, Martinez-Murillo F, Morsberger L, Lee H, Petersen D, Quackenbush J, Scott A, Wilson M, Yang Y, Ye SQ, Yu W. Multiple-laboratory comparison of microarray platforms. Nat Methods. 2005;2:345–350. doi: 10.1038/nmeth756. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C, Pounds S. False discovery rate paradigms for statistical analyses of microarray gene expression data. Bioinformation. 2007;1:436–446. doi: 10.6026/97320630001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khatri P, Desai V, Tarca AL, Sellamuthu S, Wildman DE, Romero R, Draghici S. New Onto-Tools: Promoter-Express, nsSNPCounter and Onto-Translate. Nucleic Acids Res. 2006;34:W626–631. doi: 10.1093/nar/gkl213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatri P, Sellamuthu S, Malhotra P, Amin K, Done A, Draghici S. Recent additions and improvements to the Onto-Tools. Nucleic Acids Res. 2005;33:W762–765. doi: 10.1093/nar/gki472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatri P, Voichita C, Kattan K, Ansari N, Khatri A, Georgescu C, Tarca AL, Draghici S. Onto-Tools: new additions and improvements in 2006. Nucleic Acids Res. 2007;35:W206–211. doi: 10.1093/nar/gkm327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- 22.Rajeevan MS, Vernon SD, Taysavang N, Unger ER. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J Mol Diagn. 2001;3:26–31. doi: 10.1016/S1525-1578(10)60646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 24.Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 25.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling--an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 26.Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- 27.Chen JH, Riazy M, Smith EM, Proud CG, Steinbrecher UP, Duronio V. Oxidized LDL-mediated macrophage survival involves elongation factor-2 kinase. Arterioscler Thromb Vasc Biol. 2009;29:92–98. doi: 10.1161/ATVBAHA.108.174599. [DOI] [PubMed] [Google Scholar]
- 28.Sanson M, Ingueneau C, Vindis C, Thiers JC, Glock Y, Rousseau H, Sawa Y, Bando Y, Mallat Z, Salvayre R, Negre-Salvayre A. Oxygen-regulated protein-150 prevents calcium homeostasis deregulation and apoptosis induced by oxidized LDL in vascular cells. Cell Death Differ. 2008;15:1255–1265. doi: 10.1038/cdd.2008.36. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Z, Miyoshi T, Bao Y, Sheehan JP, Matsumoto AH, Shi W. Microarray analysis of gene expression in mouse aorta reveals role of the calcium signaling pathway in control of atherosclerosis susceptibility. Am J Physiol Heart Circ Physiol. 2009;296:H1336–1343. doi: 10.1152/ajpheart.01095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Assche T, Fransen P, Guns PJ, Herman AG, Bult H. Altered Ca2+ handling of smooth muscle cells in aorta of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Cell Calcium. 2007;41:295–302. doi: 10.1016/j.ceca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Hill BJ, Price EM, Dixon JL, Sturek M. Increased calcium buffering in coronary smooth muscle cells from diabetic dyslipidemic pigs. Atherosclerosis. 2003;167:15–23. doi: 10.1016/s0021-9150(02)00381-7. [DOI] [PubMed] [Google Scholar]
- 32.Massaeli H, Austria JA, Pierce GN. Overexpression of SERCA2 Atpase in vascular smooth muscle cells treated with oxidized low density lipoprotein. Mol Cell Biochem. 2000;207:137–141. doi: 10.1023/a:1007075121729. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Hauser ER, Shah SH, Pericak-Vance MA, Haynes C, Crosslin D, Harris M, Nelson S, Hale AB, Granger CB, Haines JL, Jones CJ, Crossman D, Seo D, Gregory SG, Kraus WE, Goldschmidt-Clermont PJ, Vance JM. Peakwide mapping on chromosome 3q13 identifies the kalirin gene as a novel candidate gene for coronary artery disease. Am J Hum Genet. 2007;80:650–663. doi: 10.1086/512981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne BD, Hauser ER, Wang L, Muhlestein JB, Anderson JL, Carlquist JF, Shah SH, Kraus WE. Validation study of genetic associations with coronary artery disease on chromosome 3q13-21 and potential effect modification by smoking. Ann Hum Genet. 2009;73:551–558. doi: 10.1111/j.1469-1809.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci U S A. 1990;87:16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recinos A, 3rd, Carr BK, Bartos DB, Boldogh I, Carmical JR, Belalcazar LM, Brasier AR. Liver gene expression associated with diet and lesion development in atherosclerosis-prone mice: induction of components of alternative complement pathway. Physiol Genomics. 2004;19:131–142. doi: 10.1152/physiolgenomics.00146.2003. [DOI] [PubMed] [Google Scholar]
- 37.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human endothelial cells (EA.hy926) and mouse primary aortic endothelial cells obtained from C57BL/6 and LDb mice were plated onto chamber slides overnight. The cells were incubated with DiI-Acetyl LDL (10 μg/ml) for 6 h, and mounted with mounting media containing DAPI. The images were examined using Texas Red and DAPI filter sets at 10X magnification. DiI-Acetyl-LDL was taken up by EA.hy926 cells and primary aortic endothelial cells from C57BL/6 and LDb mice.
Human aortic smooth muscle cells (AoSMC), EA.hy926 cells, and primary aortic endothelial cells obtained from C57BL/6 and LDb mice were plated onto chamber slides overnight. The cells were hybridized with anti-CD31 at 1:100 (left panel) or anti-α-Actin at 1:100 (right panel), followed by detection using Alexa-594 conjugated secondary goat anti-rabbit IgG. The images were examined using Zeiss Observer.D1m fluorescence microscope with Texas Red and DAPI filter sets at 10× and 40× magnification. EA.hy926 cells and primary aortic endothelial cells from C57BL/6 and LDb mice stained positive for CD31, whereas AoSMC were negative for CD31. And, only AoSMC stained positive for -α-Actin, but not EA.hy926 or primary aortic endothelial cells of C57BL/6 and LDb.
Pathway Express was used to calculate and curate 30 genes that were over-represented in the calcium signaling pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG). The up-regulated genes are shown in red and the down-regulated genes are shown in blue. The green color is the default color of the pathway.