Abstract
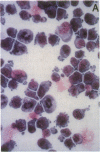
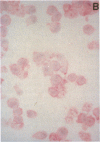
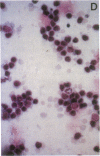
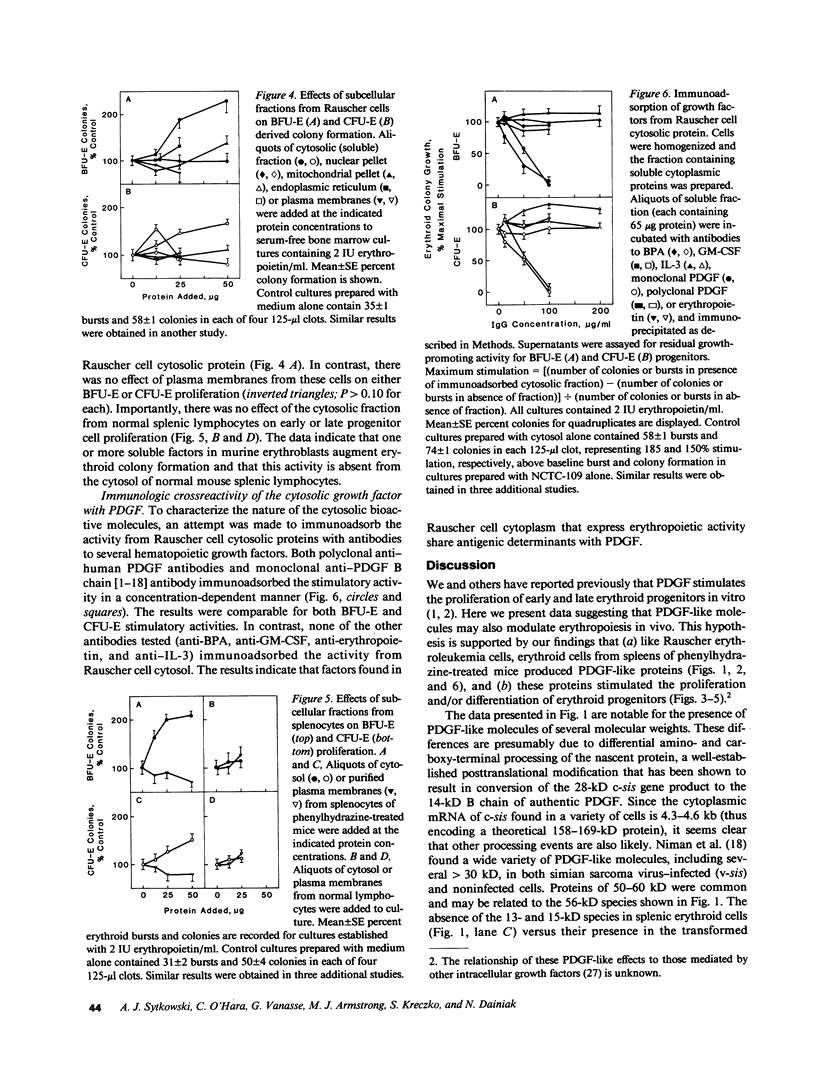
Platelet-derived growth factor (PDGF) is an important serum regulator of erythropoiesis in vitro. We have now obtained evidence suggesting that PDGF-like molecules may also modulate erythropoiesis in vivo. Western blot analysis of cytoplasmic extracts from Rauscher murine erythroleukemia cells and phenylhydrazine-treated mouse splenic erythroid cells revealed the presence of several PDGF-like proteins. The presence of PDGF-like proteins in the cytoplasm of these two erythroid cell types was confirmed by immunohistochemical staining. Using a serum-free biologic assay, PDGF-like biological activity was found in cell lysates and conditioned medium of both Rauscher cells and phenylhydrazine-treated mouse erythroid cells. Subcellular localization experiments revealed the biological activity to be concentrated in the cytosolic fraction. Using a series of antibodies to hematopoietic growth factors we demonstrated that PDGF-like biological activity was specifically immunoprecipitated by both monoclonal and polyclonal anti-human PDGF antibodies but not by antibodies to burst-promoting activity, granulocyte-macrophage colony-stimulating factor, IL-3, or erythropoietin. Taken together, the data are consistent with the hypothesis that PDGF-like molecules play a role in the regulation of mammalian erythropoiesis in vivo.
Full text
PDF




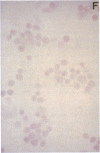
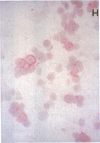
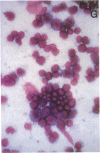
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bleiberg I., Harvey A. K., Smale G., Grotendorst G. R. Identification of a PDGF-like mitoattractant produced by NIH/3T3 cells after transformation with SV40. J Cell Physiol. 1985 May;123(2):161–166. doi: 10.1002/jcp.1041230203. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S., Wojchowski D. M., Sytkowski A. J. Erythropoietin rapidly alters phosphorylation of pp43, an erythroid membrane protein. J Biol Chem. 1987 Mar 5;262(7):2933–2936. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Feldman L., Cohen C. M. Neutralization of erythroid burst-promoting activity in vitro with antimembrane antibodies. Blood. 1985 Apr;65(4):877–885. [PubMed] [Google Scholar]
- Dainiak N., Kreczko S., Cohen A., Pannell R., Lawler J. Primary human marrow cultures for erythroid bursts in a serum-substituted system. Exp Hematol. 1985 Nov;13(10):1073–1079. [PubMed] [Google Scholar]
- Delwiche F., Raines E., Powell J., Ross R., Adamson J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J Clin Invest. 1985 Jul;76(1):137–142. doi: 10.1172/JCI111936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid J., Ebert R. F., Gesell M. S., Spivak J. L. Intracellular growth factors in polycythemia vera and other myeloproliferative disorders. Proc Natl Acad Sci U S A. 1987 Jan;84(2):532–536. doi: 10.1073/pnas.84.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Dainiak N. In vitro release of physically separable factors from monocytes that exert opposing effects on erythropoiesis. Blood. 1986 May;67(5):1454–1459. [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Riordan M. A., Dainiak N. Purification of a membrane-derived human erythroid growth factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6775–6779. doi: 10.1073/pnas.84.19.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett M., Seed T. M., Jamieson G. A. Isolation and characterization of plasma membranes and intact nuclei from lymphoid cells. J Biol Chem. 1977 Mar 25;252(6):2134–2142. [PubMed] [Google Scholar]
- Johnston J. G., van der Kooy D. Protooncogene expression identifies a transient columnar organization of the forebrain within the late embryonic ventricular zone. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1066–1070. doi: 10.1073/pnas.86.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. F., Farley B. A., Giuliano R., Kosciolek B. A., La Bella S., Rowley P. T. Induction of megakaryocytic characteristics in human leukemic cell line K562: polyploidy, inducers, and secretion of mitogenic activity. J Biol Regul Homeost Agents. 1987 Apr-Jun;1(2):73–80. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Bowen-Pope D. F. Detection of high molecular weight forms of platelet-derived growth factor by sequence-specific antisera. Science. 1984 Nov 9;226(4675):701–703. doi: 10.1126/science.6494905. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Raines E., Collins S., Nakamoto B., Tweeddale M., Ross R. Constitutive and inducible secretion of platelet-derived growth factor analogs by human leukemic cell lines coexpressing erythroid and megakaryocytic markers. J Clin Invest. 1987 Mar;79(3):859–866. doi: 10.1172/JCI112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Gullick W. J., Waterfield M. D., Rozengurt E. Highly purified fibroblast-derived growth factor, an SV40-transformed fibroblast-secreted mitogen, is closely related to platelet-derived growth factor. EMBO J. 1985 Aug;4(8):1945–1949. doi: 10.1002/j.1460-2075.1985.tb03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Fisher J. W. Isolation and characterization of an anti-peptide monoclonal antibody to human erythropoietin. J Biol Chem. 1985 Nov 25;260(27):14727–14731. [PubMed] [Google Scholar]
- Sytkowski A. J., Salvado A. J., Smith G. M., McIntyre C. J., deBoth N. J. Erythroid differentiation of clonal Rauscher erythroleukemia cells in response to erythropoietin or dimethyl sulfoxide. Science. 1980 Oct 3;210(4465):74–76. doi: 10.1126/science.6932101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- de Both N. J., Vermey M., van't Hull E., Klootwijk-van-Dijke E., van Griensven L. J., Mol J. N., Stoof T. J. A new erythroid cell line induced by Rauscher murine leukaemia virus. Nature. 1978 Apr 13;272(5654):626–628. doi: 10.1038/272626a0. [DOI] [PubMed] [Google Scholar]