Phytohemagglutinin (PHA), a mucoprotein derived from the red kidney bean, Phaseolus vulgaris, has erythroagglutinating and leukoagglutinating properties and stimulates the small lymphocytes of the peripheral blood to transform into large blast cells.4, 5, 16, 18 When administered parenterally, it can suppress the immune response in animals, if treatment is started several days before the antigenic stimulation.7, 8, 10, 12, 15, 18, 19
To our knowledge 6 studies have been reported concerning the effect of PHA on the survival of skin or renal homografts, with conflicting results.2, 3, 6, 13, 14, 17 Calne and associates2 and Casciani and Cortesini3 treated canine recipients of renal allografts solely with PHA and found a slight immunosuppressive activity; the effect was additive to that of azathioprine if the 2 agents were given together. Two of 4 publications concerned with skin grafts in rodents and rabbits reported no detectable effect of PHA,6,13 but the other 2 claimed slight prolongation of skin viability.15,17
In our laboratory the immunosuppressive qualities of a particular batch of PHA were evaluated by the ability of this agent to blunt humoral antibody formation in rabbits, to suppress the development of delayed hypersensitivity reactions in guinea pigs, and to mitigate the rejection of renal homografts in dogs. In addition the effect of red cell absorption on the biologic activity of PHA was assessed.
METHODS
Effect of absorption
The preparation and characteristics of this PHA batch* were described by Hurn,11 who reported that it was able to agglutinate both red and white blood cells. An attempt was made in the present study to remove the hemagglutinins by doubly absorbing 1 volume of reconstituted PHA solution with 1.5 volumes of packed canine red blood cells. On both occasions the mixture was incubated at 4 °C. for 30 minutes.
The effect of the procedure was quantitated in 2 ways. The first was by measurement of the hemagglutinin and leukoagglutinin titers of the PHA solution before and after absorption with red blood cells, making use of standard twofold dilution techniques. For both examinations 0.1 ml. reconstituted PHA was the original volume from which dilution was begun; 0.1 ml. of a 2 percent suspension of triply washed canine red blood cells or, alternatively, 0.1 ml. of a canine leukocyte suspension (corrected to 5,000 per cubic millimeter) was added at each dilution and the presence of agglutination determined microscopically.
Second, both the absorbed and unabsorbed PHA solutions were tested for their mitogenic effect on the lymphocytes of human peripheral blood. Human instead of canine white blood cells were used because they are so much more easily grown in culture. The cultures were adjusted to contain 106 lymphocytes and various doses of the PHA solution were instilled. After 69 hours, 3H-thymidine (2 μc per tube) was added. The cells were harvested 3 hours later. The results of thymidine uptake were expressed as mean counts per minute per tube.
Tests of immunosuppression
Humoral antibody
Four rabbits of about 2.5 kilograms were immunized with 1 ml. per kilogram of 20 percent sheep red blood cells (SRBC) intravenously on Day 0. They were treated daily with 1 ml. per kilogram unabsorbed PHA intraperitoneally from Day –2 to Day 4. Another 4 rabbits of similar weight were immunized with the same amount of SRBC on Days 0 and 10 and were treated with PHA for 7 days starting on Day 8. As control animals 5 rabbits were immunized with SRBC on Days 0 and 10 and received no treatment. In all 3 groups hemagglutinin titrations were performed every other day beginning Day –2.
Delayed hypersensitivity
Twenty-five guinea pigs weighing 600 to 700 grams were immunized subcutaneously on Days 0 and 7 with a 0.25 mg. water-in-oil suspension of killed tubercle bacilli.* Thirty-four days after the initial immunization, the guinea pigs were divided into 4 groups for single intra-peritoneal injections as follows: 5 animals, 10 mg. unabsorbed PHA; 5 animals, 5 mg. unabsorbed PHA; 4 animals, 2.5 mg. unabsorbed PHA; and 7 animals, 2.5 mg. absorbed PHA. A fifth group consisted of 4 animals which were untreated control animals.
Two days after the injections, each of the guinea pigs was challenged intracutaneously with 0.1 ml. of the standard strengths of purified protein derivatives (PPD). Induration at the PPD inoculation sites was measured after 24 to 48 hours.
Canine renal transplantation
Pelvic renal transplantation was performed in adult unrelated mongrel donors and recipients with concomitant removal of both kidneys of the recipient. Five experiments were discarded because of vascular thromboses within the first 6 days. The animals had biweekly determinations of the leukocyte count, peripheral smear, hematocrit, and BUN. At autopsy, the renal homograft, spleen, liver, lung, heart, and mesenteric lymph nodes were examined. In the test animals, the PHA was given subcutaneously each day beginning on Day –2 and continuing for at least 2 weeks in animals which survived that long. There were 4 experimental groups. Eleven animals received no treatment; 8 animals, 2.5 mg. per kilogram unabsorbed PHA per day; 6 animals, 2.5 mg. per kilogram absorbed PHA per day; and 6 animals, 5.0 mg. per kilogram absorbed PHA per day.
RESULTS
Effects of absorption
Titers
The unabsorbed PHA solution (10 mg. per milliliter) had hemagglutinin and leukoagglutinin titers of 1:256 and 1:512. After double absorption with red blood cells, these declined to 1:8 and 1:256, respectively.
Mitogenic activity
The unabsorbed PHA killed all cells at a dose of 1 mg., whereas the same quantity of the absorbed material both killed and stimulated (Table I). In smaller amounts (particularly well seen with 0.001 mg.), the unabsorbed PHA was many times more potent in stimulating blast transformation. Thus the absorption process had significantly reduced the mitogenic quality.
Table I.
3H-thymidine uptake of tissue-cultured human peripheral lymphocytes
| Dose of PHA in mg./tube | Control (c.p.m.) | Nonabsorbed (c.p.m.) | Absorbed (c.p.m.) |
|---|---|---|---|
| 0 | 865 | ||
| 1.0 | 87 | 24,690 | |
| 0.1 | 6,335 | 90,230 | |
| 0.01 | 76,950 | 118,780 | |
| 0 | 1,070 | ||
| 0.01 | 50,350 | 72,020 | |
| 0.001 | 58,760 | 4,700 | |
| 0.0001 | 1,645 | 1,245 |
Tests of immunosuppression
Humoral antibody
In the rabbits the unabsorbed PHA suppressed but did not abolish the primary hemoagglutinin response to intravenous SRBC (Fig. 1). However, there was no effect whatever on the response to rechallenge when PHA was started 2 days before a supplementary dose of SRBC was given to a previously exposed animal.
Fig. 1.
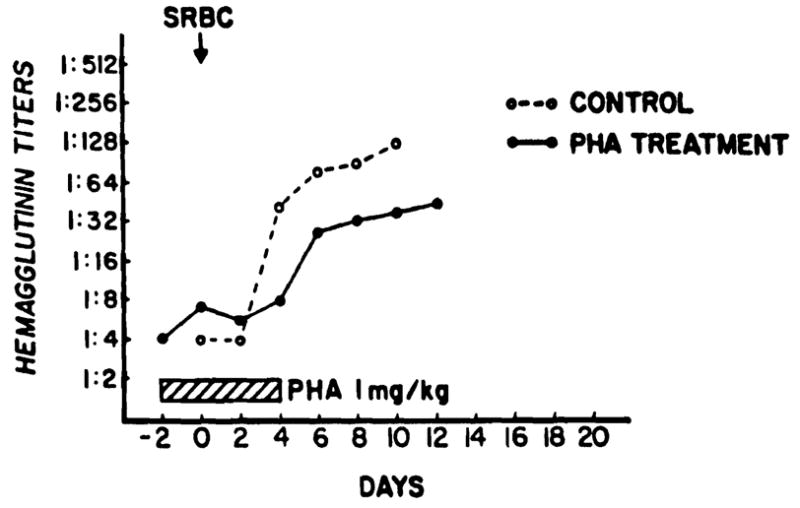
The suppressive effect of unabsorbed PHA on the hemagglutinin response of rabbit immunized intravenously with sheep red blood cells (SRBC). The results in the 2 groups were pooled from 4 PHA-treated and 5 control animals.
Delayed hypersensitivity
Unabsorbed PHA in single 10 and 5 mg. injections intraperitoneally caused a 60 percent mortality rate within 48 hours (Table II). With 2.5 mg. no deaths occurred with either the absorbed or unabsorbed PHA. At all the PHA dose levels, the expression of delayed hypersensitivity to tuberculin was blunted or blocked compared with that in the control animals (Table II). There were no significant tuberculin reactions with the first and second strength PPD injections, although 3 of the 15 PHA-treated animals developed skin in-duration of 5 mm. or more at the site of the full strength PPD inoculation.
Table II.
The toxicity and immunosuppressive effect of PHA in guinea pigs previously immunized with killed tubercle bacilli
| Group | 48 hour mortality rate of PHA | Frequency of positive PPD skin tests (intermediate strength)* |
|
|---|---|---|---|
| 24 hours | 48 hours | ||
| 10 mg.† | 3/5 | 0/2 | 0/2 |
| 5 mg.† | 3/5 | 0/2 | 0/2 |
| 2.5 mg.† | 0/4 | 0/4 | 0/4 |
| 2.5 mg.‡ | 0/7 | 0/7 | 0/7 |
| Control animals | 3/4 | 3/4 | |
A positive skin test was defined induration of 5 mm. or greater At full strength PPD, 3 of the 15 PHA-treated animals had positive skin tests as did all 4 of the control animals.
Unabsorbed.
Absorbed.
Canine renal transplantation
The PHA was toxic. Anorexia, diarrhea (often bloody), and weight loss were seen in most of the dogs. Some of the animals had remarkable swelling of the extremities in which the subcutaneous injections were given. Anemia was almost universal (Fig. 2). All the foregoing side effects were most severe and common in the dogs treated with unabsorbed PHA.
Fig. 2.
The anemia seen in dogs subjected to renal homotransplantation and treated with either absorbed or unabsorbed PHA. Note that the hematocrit falls were most pronounced in the animals injected with unabsorbed PHA. The animals that contributed to each study group are the same as in Table III.
Treatment with PHA invariably induced a leukocytosis in the pretransplantation period, with the appearance of immature forms of all cell types. After transplantation there was no significant difference in the lymphocyte counts in the control animals as compared with those treated with PHA.
The results in these experiments are summarized in Table III. At doses of 2.5 mg. per kilogram per day, the animals receiving the unabsorbed PHA had a significantly prolonged survival (p < 0.01) whereas those treated with the absorbed material did not. The best results were with 5 mg. per kilogram per day of absorbed PHA (Table III). It was necessary to stop the injections in 3 dogs in the latter group 16, 23, and 32 days after transplantation because of a shortage of PHA. The dogs died of uremia 17,29, and 8 days later (Fig. 3). On histologic examination, there were no distinguishing features of the homografts retrieved from the treated dogs as compared with those from the control animals. Both had classical evidence of rejection.
Table III.
The effect of absorbed and unabsorbed PHA on survival after canine renal transplantation
| Group | No. of dogs | Survival (days) | Range (days) | p |
|---|---|---|---|---|
| Control animals | 11 | 9.0 ± 1.7 (S.E.) | 4 to 21 | |
| 2.5 mg./kg.* | 8 | 16.3 ± 2.3 (S.E.) | 8 to 27 | <0.01 |
| 2.5 mg./kg.† | 6 | 10.2 ± 1.3 (S.E.) | 6 to 14 | |
| 5.0 mg./kg.† | 6 | 25.4 ± 7.8 (S.E.) | 5 to 52 | <0.01 |
Unabsorbed.
Absorbed.
Fig. 3.
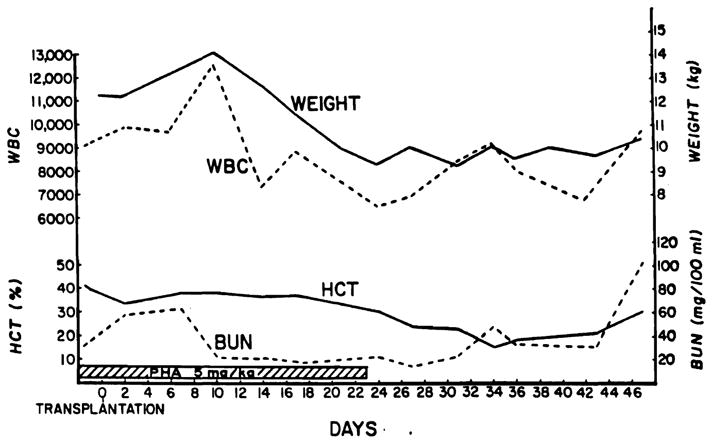
The course of a dog after a renal homotransplantation from a nonrelated mongrel donor. Treatment was with 5 mg. per kilogram per day absorbed PHA; injections were stopped after 23 days. The dog developed rejection and died 52 days after operation. Note the progressive anemia and weight loss.
In the spleens of the animals treated with PHA, there was generally a reduced cellularity in the red pulp. Contrary to what has been reported in mice,9 the white pulp was not hyperplastic and, if anything, seemed reduced in mass; this was particularly true if unabsorbed PHA had been given.
Extramedullary hematopoiesis in both the spleen and liver was common in the PHA-treated animals, especially prominently in those which had received the unabsorbed material and had developed severe anemia. Mild intrahepatic cholestasis was present in the livers of several of the same dogs.
The most disquieting extrarenal finding was myocardial necrosis in 3 of the 12 PHA-treated animals from which the heart was examined histologically. The infarctions were of moderate size, were not transmural, and appeared to be 3 or 4 days old. The significance of these abnormalities is obscured by the fact that similar lesions can be caused in dogs by other kinds of trauma.20
DISCUSSION
The results of the present study add to the growing body of evidence that PHA is an immunosuppressive agent of intermediate effectiveness. Its ability to suppress a humoral antibody response has been most widely accepted7, 8, 10, 12, 15, 18 and has been confirmed in our experiments. The striking way in which PHA partially or completely prevents the expression of a pre-existing state of delayed hypersensitivity has not been generally appreciated, the only other description of this effect being that of Stevens and Willoughby.19
Unfortunately, the side effects of the relatively unrefined PHA used in the present study were too severe to permit its use in clinical medicine. A number of deaths were caused in average-sized guinea pigs with a single intraperitoneal injection of 5 or 10 mg. In dogs given 2.5 or 5 mg. per kilogram, severe local reactions at the sites of injection were common. With continued treatment the animals became clinically ill. Anemia was often severe.
Hope that an improved PHA might be developed derives from the fact that some of the toxic properties can be largely removed by simple absorption technique. The investigations of Barkhan and Ballas1 suggested that the leukoagglutinating and hemagglutinating activities of PHA were not closely interrelated. This observation was exploited in the present experiments by abosorbing most of the hemagglutinating properties with red blood cells.
In doing so, there was a concomitant loss of some of the leukoagglutinating and mitogenic potential of the PHA solution as judged by both in vitro and in vivo testing. This necessitated upward dose adjustments in the transplantation experiments. Nevertheless, the absorbed PHA seemed to have an improved therapeutic index.
SUMMARY
Phytohemagglutinin (PHA) has been shown to inhibit the hemagglutinin response of rabbits to intravenous sheep red blood cells, to suppress the expression of pre-existing tuberculin sensitivity in guinea pigs, and to prolong the functional survival of renal homograft in dogs. Concomitant with these desired effects are a number of side reactions; sudden death can be caused in guinea pigs and in dogs, and anemia and wasting can be induced. The toxicity of PHA can be partly reduced by preliminary absorption with red cells.
Acknowledgments
Supported by United States Public Health Service Grants AM-06344, HE-07735, AM-07772, FR-00051, AI-04152, FR-00069, AM-12148, and AI-AM-06896.
Footnotes
The desirability of performing this study was suggested to us by Dr. Maurice Landy of Bethesda, Md. He also assisted in formulating the experimental protocol. The PHA used, Phytoclin Batch E313, was obtained by Dr. George Hitchings from the Wellcome Research Laboratories, Beckenham, England. The desiccated PHA was reconstituted by adding 1 ml. normal saline to 10 mg. of the powder before use.
Three other control animals were inoculated with a water-in-oil suspension without killed tubercle bacilli. They did not become purified protein derivative–positive.
References
- 1.Barkhan P, Ballas A. Phytohaemagglutinin: Separation of haemagglutinating and mitogenic principles. Nature. 1963;200:141. doi: 10.1038/200141a0. [DOI] [PubMed] [Google Scholar]
- 2.Calne RY, Wheeler JR, Hurn BAL. Combined immunosuppressive action of phytohaemmagglutinin and azothioprine (Imuran) on dogs with renal homografts. Brit M J. 1965;2:154. doi: 10.1136/bmj.2.5454.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casciani C, Cortesini R. Phytohemagglutinin action of renal homograft survival. In: Curtoni ES, editor. Histocompatibility testing. Baltimore: The Williams & Wilkins Company; 1967. p. 83. [Google Scholar]
- 4.Claman HN, Brunstetter FH. Effects of antilymphocyte serum and phytohaemagglutinin upon cultures of human thymus and peripheral blood lymphoid cells. I. Morphologic and biochemical studies of thymus and blood lymphoid cells. Internat Acad Path. 1968;18:757. [PubMed] [Google Scholar]
- 5.Cooper HL, Rubin AD. RNA metabolism in lymphocytes stimulated by phytohemagglutinin: Initial responses to phytohemagglutinin. Blood. 1965;25:1014. [PubMed] [Google Scholar]
- 6.Elves MW. The in vivo effect of phytohemagglutinin on homograft reactions. Transplantation. 1967;5:1532. [Google Scholar]
- 7.Elves MW. On the mechanism of action of phytohaemagglutinin on immunological reactions. Internat Arch Allergy. 1968;33:353. doi: 10.1159/000230052. [DOI] [PubMed] [Google Scholar]
- 8.Elves MW. Suppression of antibody production by phytohaemagglutinin. Nature. 1967;213:495. doi: 10.1038/213495a0. [DOI] [PubMed] [Google Scholar]
- 9.Gamble CN. The effects of PHA on mouse spleen cells in vivo. Blood. 1966;28:175. [PubMed] [Google Scholar]
- 10.Gengozion N, Huebner KF. Effect of phytohemagglutinin (PHA) on an antibody-forming system. J Immunol. 1967;99:184. [PubMed] [Google Scholar]
- 11.Hurn BAL. Preparation and partial characterisation of the mitogenic principle from Phaseolus vulgaris seeds. In: Elves MW, editor. The biological effects of phytohaemagglutinin; Proc. Symposium; Oswestry, England. The Robert Jones and Agnes Hunt Orthopaedic Hospital Management Committee; 1967. p. 83. [Google Scholar]
- 12.Jennings JF, Oates CM. The effect of phytohaemagglutinin on the immune response in vivo. Clin Exper Immunol. 1967;2:445. [PMC free article] [PubMed] [Google Scholar]
- 13.Kehn B, Rigby P. Effects of phytohaemagglutinin on homograft rejection. Nature. 1967;217:182. doi: 10.1038/216182b0. [DOI] [PubMed] [Google Scholar]
- 14.Markley K, Evans G, Smallman E. Prolongation of skin allograft survival time by phytohaemagglutinin. Transplantation. 1967;5:1535. [Google Scholar]
- 15.Markley K, Smallman E, Evans G. Decreased mouse circulating antibodies to sheep erythrocytes by phytohaemagglutinin. Internat Arch Allergy. 1967;32:482. doi: 10.1159/000229959. [DOI] [PubMed] [Google Scholar]
- 16.Nowell PC. Phytohemagglutinin: An initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462. [PubMed] [Google Scholar]
- 17.St Pierre RL, Younger JB, Zmijewski CM. Effect of phytohemagglutinin on skin allograft survival in mice. Proc Soc Exper Biol & Med. 1967;126:687. [Google Scholar]
- 18.Spreafico F, Lerner EM. II: Suppression of the primary and secondary immune response on the mouse by phytohaemagglutinin. J Immunol. 1967;98:407. [PubMed] [Google Scholar]
- 19.Stevens JE, Willoughby DA. Phytohaemagglutinin and cell mediated hypersensitivity reactions in rat. Nature. 1967;215:967. doi: 10.1038/215967a0. [DOI] [PubMed] [Google Scholar]
- 20.Tanturi C, Mejia RH, Canepa JF, Gomez OT. Electrocardiographic and humoral changes in transient occlusion of the portal vein in the dog. Surg Gynec & Obst. 1960;110:537. [PubMed] [Google Scholar]



