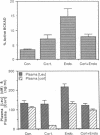
Abstract
Protein catabolic states (i.e., sepsis and trauma) are thought to be associated with accelerated oxidation of branched-chain amino acids (BCAA). Branched-chain alpha-keto acid dehydrogenase (BCKAD), the rate-limiting enzyme for BCAA oxidation by muscle, is regulated by phosphorylation/dephosphorylation. Skeletal muscle BCKAD was only 2-4% active in control rats. Intravenous injection of Salmonella enteritidis endotoxin (0.25-10 mg/kg) did not change total BCKAD activity, but increased the percent active enzyme in muscle three- to four-fold in 4-6 h. Identical results were observed in adrenalectomized rats pretreated with one dose of alpha-methylprednisolone (2.5 mg/kg i.p.) 30-60 min before saline or endotoxin injection, indicating that endotoxin's effect was not mediated by hypersecretion of adrenal hormones. Cortisone pretreatment of normal rats (100 mg/kg per d) for 2 d prevented endotoxin-induced activation of muscle BCKAD, suggesting that endogenous secretion products mediated BCKAD activation by endotoxin. Human recombinant tumor necrosis factor-alpha and/or IL-1 beta or alpha (50 micrograms/kg) increased muscle BCKAD activation two- to fourfold in normal rats 4-6 h after intravenous injection. We conclude that cytokine-mediated activation of muscle BCKAD may contribute to accelerated BCAA oxidation in septicemia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Block K. P., Buse M. G. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986 May;250(5 Pt 1):E599–E604. doi: 10.1152/ajpendo.1986.250.5.E599. [DOI] [PubMed] [Google Scholar]
- Aftring R. P., Manos P. N., Buse M. G. Catabolism of branched-chain amino acids by diaphragm muscles of fasted and diabetic rats. Metabolism. 1985 Aug;34(8):702–711. doi: 10.1016/0026-0495(85)90018-6. [DOI] [PubMed] [Google Scholar]
- Aftring R. P., Miller W. J., Buse M. G. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am J Physiol. 1988 Mar;254(3 Pt 1):E292–E300. doi: 10.1152/ajpendo.1988.254.3.E292. [DOI] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Beisel W. R., Wannemacher R. W., Jr Gluconeogenesis, ureagenesis, and ketogenesis during sepsis. JPEN J Parenter Enteral Nutr. 1980 May-Jun;4(3):277–285. doi: 10.1177/014860718000400307. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Block K. P., Aftring R. P., Mehard W. B., Buse M. G. Modulation of rat skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Effects of dietary protein and meal consumption. J Clin Invest. 1987 May;79(5):1349–1358. doi: 10.1172/JCI112961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K. P., Richmond W. B., Mehard W. B., Buse M. G. Glucocorticoid-mediated activation of muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1987 Mar;252(3 Pt 1):E396–E407. doi: 10.1152/ajpendo.1987.252.3.E396. [DOI] [PubMed] [Google Scholar]
- Brennan M. F., Cerra F., Daly J. M., Fischer J. E., Moldawer L. L., Smith R. J., Vinnars E., Wannemacher R., Young V. R. Report of a research workshop: branched-chain amino acids in stress and injury. JPEN J Parenter Enteral Nutr. 1986 Sep-Oct;10(5):446–452. doi: 10.1177/0148607186010005446. [DOI] [PubMed] [Google Scholar]
- Cerra F. B., Siegel J. H., Coleman B., Border J. R., McMenamy R. R. Septic autocannibalism. A failure of exogenous nutritional support. Ann Surg. 1980;192(4):570–580. doi: 10.1097/00000658-198010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Randall H. T., Cha C. J. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Duff J. H., Viidik T., Marchuk J. B., Holliday R. L., Finley R. J., Groves A. C., Woolf L. I. Femoral arteriovenous amino acid differences in septic patients. Surgery. 1979 Mar;85(3):344–348. [PubMed] [Google Scholar]
- Flores E. A., Bistrian B. R., Pomposelli J. J., Dinarello C. A., Blackburn G. L., Istfan N. W. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest. 1989 May;83(5):1614–1622. doi: 10.1172/JCI114059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Goldberg A. L., Kettelhut I. C., Furuno K., Fagan J. M., Baracos V. Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin 1 or other known monokines. J Clin Invest. 1988 May;81(5):1378–1383. doi: 10.1172/JCI113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. C., Woolf L. I., Duff J. H., Finley R. J. Metabolism of branched-chain amino acids in dogs with Escherichia coli endotoxin shock. Surgery. 1983 Feb;93(2):273–278. [PubMed] [Google Scholar]
- Harper A. E., Miller R. H., Block K. P. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Hood D. A., Terjung R. L. Leucine metabolism in perfused rat skeletal muscle during contractions. Am J Physiol. 1987 Dec;253(6 Pt 1):E636–E647. doi: 10.1152/ajpendo.1987.253.6.E636. [DOI] [PubMed] [Google Scholar]
- Hutson S. M. Branched chain alpha-keto acid oxidative decarboxylation in skeletal muscle mitochondria. Effect of isolation procedure and mitochondrial delta pH. J Biol Chem. 1986 Apr 5;261(10):4420–4425. [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperek G. J., Dohm G. L., Snider R. D. Activation of branched-chain keto acid dehydrogenase by exercise. Am J Physiol. 1985 Feb;248(2 Pt 2):R166–R171. doi: 10.1152/ajpregu.1985.248.2.R166. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettelhut I. C., Goldberg A. L. Tumor necrosis factor can induce fever in rats without activating protein breakdown in muscle or lipolysis in adipose tissue. J Clin Invest. 1988 May;81(5):1384–1389. doi: 10.1172/JCI113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Ishizaki M., Nemoto M., Nakamura T., Suzuki M. Antishock effects of corticosterone on dextran-induced shock in rats. Am J Physiol. 1986 Nov;251(5 Pt 1):E569–E575. doi: 10.1152/ajpendo.1986.251.5.E569. [DOI] [PubMed] [Google Scholar]
- Lee M. D., Zentella A., Vine W., Pekala P. H., Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987 May;84(9):2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loda M., Clowes G. H., Jr, Dinarello C. A., George B. C., Lane B., Richardson W. Induction of hepatic protein synthesis by a peptide in blood plasma of patients with sepsis and trauma. Surgery. 1984 Aug;96(2):204–213. [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. E., Buse M. G. Effects of branched-chain amino acids on protein turnover. Diabetes Metab Rev. 1989 May;5(3):227–245. doi: 10.1002/dmr.5610050303. [DOI] [PubMed] [Google Scholar]
- May R. C., Hara Y., Kelly R. A., Block K. P., Buse M. G., Mitch W. E. Branched-chain amino acid metabolism in rat muscle: abnormal regulation in acidosis. Am J Physiol. 1987 Jun;252(6 Pt 1):E712–E718. doi: 10.1152/ajpendo.1987.252.6.E712. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Lowry S. F., Cerami A. Cachectin: its impact on metabolism and nutritional status. Annu Rev Nutr. 1988;8:585–609. doi: 10.1146/annurev.nu.08.070188.003101. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Svaninger G., Gelin J., Lundholm K. G. Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol. 1987 Dec;253(6 Pt 1):C766–C773. doi: 10.1152/ajpcell.1987.253.6.C766. [DOI] [PubMed] [Google Scholar]
- Moyer E. D., McMenamy R. H., Cerra F. B., Reed R. A., Yu L., Chenier R., Caruana J., Border J. R. Multiple systems organ failure: III Contrasts in plasma amino acid profiles in septic trauma patients who subsequently survive and do not survive-effects of intravenous amino acids. J Trauma. 1981 Apr;21(4):263–274. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnel T. F., Clowes G. H., Jr, Blackburn G. L., Ryan N. T., Benotti P. N., Miller J. D. Proteolysis associated with a deficit of peripheral energy fuel substrates in septic man. Surgery. 1976 Aug;80(2):192–200. [PubMed] [Google Scholar]
- Pomposelli J. J., Flores E. A., Bistrian B. R. Role of biochemical mediators in clinical nutrition and surgical metabolism. JPEN J Parenter Enteral Nutr. 1988 Mar-Apr;12(2):212–218. doi: 10.1177/0148607188012002212. [DOI] [PubMed] [Google Scholar]
- Pomposelli J. J., Palombo J. D., Hamawy K. J., Bistrian B. R., Blackburn G. L., Moldawer L. L. Comparison of different techniques for estimating rates of protein synthesis in vivo in healthy and bacteraemic rats. Biochem J. 1985 Feb 15;226(1):37–42. doi: 10.1042/bj2260037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofe A. M., Conyers R. A., Bais R., Gamble J. R., Vadas M. A. The effects of recombinant tumour necrosis factor (cachectin) on metabolism in isolated rat adipocyte, hepatocyte and muscle preparations. Biochem J. 1987 Nov 1;247(3):789–792. doi: 10.1042/bj2470789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt S., Clowes G. H., Jr, George B. C., Hirsch E., Lindberg B. Exchange of amino acids by muscle and liver in sepsis. Arch Surg. 1983 Feb;118(2):167–175. doi: 10.1001/archsurg.1983.01390020023004. [DOI] [PubMed] [Google Scholar]
- Ryan N. T., Blackburn G. L., Clowes H. A., Jr Differential tissue sensitivity to elevated endogenous insulin levels during experimental peritonitis in rats. Metabolism. 1974 Nov;23(11):1081–1089. doi: 10.1016/0026-0495(74)90075-4. [DOI] [PubMed] [Google Scholar]
- Ryan N. T. Metabolic adaptations for energy production during trauma and sepsis. Surg Clin North Am. 1976 Oct;56(5):1073–1090. doi: 10.1016/s0039-6109(16)41032-7. [DOI] [PubMed] [Google Scholar]
- Seelentag W. K., Mermod J. J., Montesano R., Vassalli P. Additive effects of interleukin 1 and tumour necrosis factor-alpha on the accumulation of the three granulocyte and macrophage colony-stimulating factor mRNAs in human endothelial cells. EMBO J. 1987 Aug;6(8):2261–2265. doi: 10.1002/j.1460-2075.1987.tb02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D. R., Sherman M. L., Frei E., 3rd, Kufe D. W. Clinical studies with tumour necrosis factor. Ciba Found Symp. 1987;131:206–227. doi: 10.1002/9780470513521.ch14. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocco-Bradley R., Moldawer L. L., Jones C. T., Gerson B., Blackburn G. L., Bistrian B. R. The biological activity in vivo of recombinant murine interleukin 1 in the rat. Proc Soc Exp Biol Med. 1986 Jun;182(2):263–271. doi: 10.3181/00379727-182-42338. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Beutler B., Cerami A., Albert J. D., Shires G. T. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med. 1986 Oct 1;164(4):1368–1373. doi: 10.1084/jem.164.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Cerami A. Cachectin: a hormone that triggers acute shock and chronic cachexia. J Infect Dis. 1988 Mar;157(3):413–420. doi: 10.1093/infdis/157.3.413. [DOI] [PubMed] [Google Scholar]
- Vary T. C., Siegel J. H., Nakatani T., Sato T., Aoyama H. Effect of sepsis on activity of pyruvate dehydrogenase complex in skeletal muscle and liver. Am J Physiol. 1986 Jun;250(6 Pt 1):E634–E640. doi: 10.1152/ajpendo.1986.250.6.E634. [DOI] [PubMed] [Google Scholar]
- Vary T. C., Siegel J. H., Zechnich A., Tall B. D., Morris J. G., Placko R., Jawor D. Pharmacological reversal of abnormal glucose regulation, BCAA utilization, and muscle catabolism in sepsis by dichloroacetate. J Trauma. 1988 Sep;28(9):1301–1311. [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Gabrilove J. L., Oettgen H. F., Brennan M. F. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987 Dec;122(12):1396–1400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]
- Woolf L. I., Groves A. C., Duff J. H. Amino acid metabolism in dogs with E. coli bacteremic shock. Surgery. 1979 Feb;85(2):212–218. [PubMed] [Google Scholar]
- Ziegler E. J. Tumor necrosis factor in humans. N Engl J Med. 1988 Jun 9;318(23):1533–1535. doi: 10.1056/NEJM198806093182309. [DOI] [PubMed] [Google Scholar]