Abstract
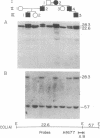
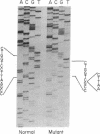
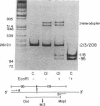
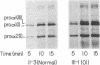
Osteogenesis imperfecta (OI) is a heterogeneous disorder of type I collagen of which OI type I, an autosomal dominant condition, is the mildest and most common form. Affected individuals have blue sclerae, normal stature, bone fragility without significant deformity and osteopenia. Fibroblasts from most affected individuals produce about half the expected amount of structurally normal type I collagen as a result of decreased synthesis of one of its constituent chains, pro alpha 1(I), but the nature of the mutations which result in OI type I are unknown. We describe a three generation family with OI type I in which all affected members have one normal COL1A1 allele and another from which the intragenic Eco RI restriction site near the 3' end of the gene is missing. Amplification by polymerase chain reaction and sequence determination of the normal allele and of the mutant allele in the domain that normally contains the Eco RI site demonstrated a 5-bp deletion from the mutant allele. The deletion changes the translational reading-frame beginning at the Eco RI site and predicts the synthesis of a pro alpha 1(I) chain that extends 84 amino acids beyond the normal termination. Although the mutant pro alpha 1(I) chain is synthesized in an in vitro translation system, we are unable to detect its presence in intact cells, suggesting that it is unstable and rapidly destroyed in one of the cell's degradative pathways. Our analysis of individuals with OI type I from 20 families indicates that this is a unique mutation and suggests that the phenotype can result from multiple mechanisms that decrease the synthesis of normal type I procollagen molecules, including those that alter protein stability.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Gelinas R. E. DNA and chromatin structure of the human alpha 1 (I) collagen gene. J Biol Chem. 1984 Dec 10;259(23):14906–14913. [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S., Chan D., Cole W. G. Peptide analysis of collagen produced from cDNA by transcription and translation in vitro. Biochem J. 1987 Jul 15;245(2):393–398. doi: 10.1042/bj2450393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Rome L. H., Crystal R. G. Lysosomal function in the degradation of defective collagen in cultured lung fibroblasts. Biochemistry. 1984 May 8;23(10):2134–2138. doi: 10.1021/bi00305a005. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J Biol Chem. 1985 Feb 25;260(4):2315–2320. [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Contopolou-Griva I., Caroutsos K., Poungouras P., Tsevrenis H. Haemoglobin Icaria, a new chain-termination mutant with causes alpha thalassaemia. Nature. 1974 Sep 20;251(5472):245–247. doi: 10.1038/251245a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Apone S., Eyre D. R., Starman B. J., Andreassen P., Charbonneau H., Nicholls A. C., Pope F. M., Byers P. H. Substitution of cysteine for glycine within the carboxyl-terminal telopeptide of the alpha 1 chain of type I collagen produces mild osteogenesis imperfecta. J Biol Chem. 1988 Oct 15;263(29):14605–14607. [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak S. B., Nicholls A., Pope F. M., Prockop D. J. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem. 1983 Dec 25;258(24):15192–15197. [PubMed] [Google Scholar]
- Derry S., Wood W. G., Pippard M., Clegg J. B., Weatherall D. J., Wickramasinghe S. N., Darley J., Fucharoen S., Wasi P. Hematologic and biosynthetic studies in homozygous hemoglobin Constant Spring. J Clin Invest. 1984 Jun;73(6):1673–1682. doi: 10.1172/JCI111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Stiegler P., Ebel J. P. Partial nucleotide sequence of 16S ribosomal RNA from E. coli. Nat New Biol. 1972 Sep 6;239(88):1–5. [PubMed] [Google Scholar]
- Genovese C., Rowe D. Analysis of cytoplasmic and nuclear messenger RNA in fibroblasts from patients with type I osteogenesis imperfecta. Methods Enzymol. 1987;145:223–235. doi: 10.1016/0076-6879(87)45012-x. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Dowling C. E., Waber P. G., Huang S., Lo W. H. The spectrum of beta-thalassemia genes in China and Southeast Asia. Blood. 1986 Oct;68(4):964–966. [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux B., Dennis D., White H. B., 3rd Human alpha-chain globin messenger: prediction of a nucleotide sequence. Biochem Biophys Res Commun. 1973 Oct 1;54(3):894–898. doi: 10.1016/0006-291x(73)90778-x. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Pope F. M., Craig D. An abnormal collagen alpha chain containing cysteine in autosomal dominant osteogenesis imperfecta. Br Med J (Clin Res Ed) 1984 Jan 14;288(6411):112–113. doi: 10.1136/bmj.288.6411.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Dickson L. A., Pope F. M., Korhonen V. R., Nicholls A., Prockop D. J., Myers J. C. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984 Nov 10;259(21):12941–12944. [PubMed] [Google Scholar]
- Rowe D. W., Shapiro J. R., Poirier M., Schlesinger S. Diminished type I collagen synthesis and reduced alpha 1(I) collagen messenger RNA in cultured fibroblasts from patients with dominantly inherited (type I) osteogenesis imperfecta. J Clin Invest. 1985 Aug;76(2):604–611. doi: 10.1172/JCI112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Nicholls A., Pope F. M. Clinical variability of osteogenesis imperfecta reflecting molecular heterogeneity: cysteine substitutions in the alpha 1(I) collagen chain producing lethal and mild forms. J Biol Chem. 1986 Jul 5;261(19):8958–8964. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Sykes B., Francis M. J., Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977 May 26;296(21):1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Valle G., Besley J., Williamson A. R., Mosmann T. R., Colman A. Post-translational fate of variant MOPC 315 lambda chains in Xenopus oocytes and mouse myeloma cells. Eur J Biochem. 1983 Apr 15;132(1):131–138. doi: 10.1111/j.1432-1033.1983.tb07337.x. [DOI] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Wallis G., Beighton P., Boyd C., Mathew C. G. Mutations linked to the pro alpha 2(I) collagen gene are responsible for several cases of osteogenesis imperfecta type I. J Med Genet. 1986 Oct;23(5):411–416. doi: 10.1136/jmg.23.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Wenstrup R. J., Hunter A. G., Byers P. H. Osteogenesis imperfecta type IV: evidence of abnormal triple helical structure of type I collagen. Hum Genet. 1986 Sep;74(1):47–53. doi: 10.1007/BF00278784. [DOI] [PubMed] [Google Scholar]
- Wenstrup R. J., Tsipouras P., Byers P. H. Osteogenesis imperfecta type IV. Biochemical confirmation of genetic linkage to the pro alpha 2(I) gene of type I collagen. J Clin Invest. 1986 Dec;78(6):1449–1455. doi: 10.1172/JCI112735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. B., 3rd, Laux B. E., Dennis D. Messenger RNA structure: compatibility of hairpin loops with protein sequence. Science. 1972 Mar 17;175(4027):1264–1266. doi: 10.1126/science.175.4027.1264. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]
- de Vries W. N., de Wet W. J. The molecular defect in an autosomal dominant form of osteogenesis imperfecta. Synthesis of type I procollagen containing cysteine in the triple-helical domain of pro-alpha 1(I) chains. J Biol Chem. 1986 Jul 5;261(19):9056–9064. [PubMed] [Google Scholar]