Abstract
Disorders of the anorectum and pelvic floor affect approximately 25% of the population. Their evaluation and treatment have been hindered by a lack of understanding of underlying mechanism(s) and a working knowledge of the diagnostic advances in this field. A meticulous evaluation of anorectal structure and its function can provide invaluable insights to the practicing gastroenterologist regarding the pathogenic mechanism(s) of these disorders. Also, significant new knowledge has emerged over the past decade that include the development of newer diagnostic tools such as high resolution manometry and MR defecography as well as a better delineation of the clinical and pathophysiological subtypes of constipation and incontinence. This article provides an up-to-date review on the role of diagnostic tests in the evaluation of fecal incontinence and constipation with dyssynergic defecation.
Keywords: Fecal incontinence, Dyssynergic defecation, Anorectal manometry, wireless motility capsule, rectal sensation, rectal compliance, anal ultrasound
INTRODUCTION
Disorders of anorectum affect one quarter of the population, and are mostly due to neuromuscular dysfunction (1). Hence, a meticulous evaluation of anorectal structure and function can provide much needed information regarding the pathophysiology of these disorders. This review provides an appraisal of the advances in the mechanistic understanding and diagnostic evaluation of fecal incontinence and dyssynergic defecation.
NEUROANATOMY AND PHYSIOLOGY OF THE ANORECTUM
Structure of the anorectum
The rectum is a muscular tube, 12 to 15 cm long that terminates as the anus. The internal anal sphincter (IAS), the external anal sphincter (EAS) and the anal vascular cushions encircle the anal orifice and together maintain continence at rest whereas the EAS and puborectalis provide the mechanical barrier during voluntary squeeze (2,3),. The puborectalis is a 0.5–1.0 cm thick “u” shaped muscle, which forms a flap-like valve that creates a forward pull and reinforces the anorectal angle. Recent work using transperineal ultrasound has demonstrated that all three muscles form the mechanical barrier (4). Furthermore, puborectalis dysfunction and injury are common even in asymptomatic woman and contribute to incontinence (4,5).
Rectoanal inhibitory reflex is a well known physiological response but recently, a desire to defecate in response to rectal distension has been shown to be associated with a unique and reproducible anal contractile response- the sensory-motor response (SMR) (6). The SMR is altered in patients with rectal hyposensitivity (7).
Rectal sensation arises from stimulation of nerve endings and mechanoreceptors in the rectal wall and adjacent structures and is transmitted along the pelvic splanchnic and S2-S4 parasympathetic nerves (2). Recent studies from rat models have confirmed the existence of intraganglionic laminar nerve endings in the myenteric plexus of the rectal wall that respond to mechanical distension (8). Cortical mapping shows that rectal and anal perceptions are bilaterally represented in the superior motor cortex, i.e. Brodmann area 4 (9).
Physiology of defecation and continence
Defecation involves several stereotypical events that are under voluntary and involuntary control. The basic regulatory mechanisms are present in the newborn but the art of controlled defecation develops through training and is controlled by higher cortical centers. Arrival of stool in the rectum causes rectal distension and induces a desire to defecate along with a decrease in anal resting pressure –the RAIR. These events allow the rectal contents to come into contact with the sensitive anoderm, and based on the nature of fecal material “sampled”, solid, liquid, or gas (18), an urge to defecate is induced that can only be resisted by vigorous contractions of the EAS and puborectalis muscle. If social conditions are favorable, the subject sits or squats, holds breath, contracts the diaphragm, abdominal and rectal muscles and simultaneously relaxes the EAS and puborectalis muscle. These maneuvers open the anus and move stool. Thus, sensory perception and coordinated movement of stool are important physiologic variables that affect anorectal function. Likewise, weakness of anal sphincter or puborectalis, neuropathy and altered rectal or anal sensation or diarrheal conditions may each overwhelm normal ability to maintain continence and result in leakage of stool.
FECAL INCONTINENCE
Fecal incontinence is the inability to control or involuntary discharge of stool or gas. It affects 8–9 % of population, and disproportionately affects middle-aged women, and nursing home residents (9–11). Advancing age, diarrhea, urinary incontinence and multiple childbirths, particularly vaginal delivery with sphincter tear are independent risk factors (9–11). It significantly affects quality of life and consumes substantial health care resources (9–11).
CLINICAL AND DIAGNOSTIC EVALUATION OF PATIENTS WITH FECAL INCONTINENCE
The evaluation includes a detailed clinical assessment together with the appropriate physiological and imaging tests of the anorectum that should provide information regarding the severity and impact of the problem and possible etiology.
Clinical Features
The first step is to establish a trustworthy relationship and to assess the duration, nature i.e., whether the leakage consists of flatus, liquid or solid stool, and its impact on the quality of life. Obstetric history, co-existing conditions such as diabetes mellitus, pelvic radiation, neurological problems or spinal cord injury, dietary history and a history of co-existing urinary incontinence is important. A prospective stool diary can be useful (12).
A detailed physical and neurological examination and digital rectal examination (DRE) is essential. Upon inspection, the presence of fecal matter, prolapsed hemorrhoids, dermatitis, scars, skin excoriation, the absence of perianal creases or a gaping anus may be noted. Excessive perineal descent (outward bulge > 3 cm) or rectal prolapse can be demonstrated by asking the patient to attempt defecation (12). The perianal sensation and anocutaneous reflex are assessed by stroking the perianal skin with a cotton bud in each quadrant. The normal response consists of a brisk contraction of the external anal sphincter. Impaired or absent anocutaneous reflex suggests neuronal injury (12). After inserting a lubricated, gloved index finger, one should assess the resting sphincter tone, the length of anal canal, the strength of the puborectalis sling, the acuteness of the anorectal angle, the strength of anal sphincter squeeze and the elevation of the perineum during voluntary squeeze. Also, the presence of rectocele or impacted stools may be noted. A recent study showed that trainees lack adequate skills for recognizing the DRE features of incontinence (13). Thus, DRE is prone to inter-observer differences and is a learned skill.
Clinical assessment should facilitate the recognition of three sometimes overlapping subtypes; passive incontinence, urge incontinence, and fecal seepage (2,12), although symptoms assessment may not always correlate with manometric findings. In one study, leakage had a sensitivity of 98.9%, specificity of 11% and positive predictive value of 51% for detecting low resting sphincter pressure (14). The positive predictive value for detecting a low squeeze pressure was 80% (14). In another study, adequate correlation was reported between DRE and manometry (15). Thus, history and clinical features alone may be insufficient to define the pathophysiology and objective testing is essential (10,12). Several methods are available for grading the severity of fecal incontinence and its quality of life (16).
INVESTIGATIONS OF FECAL INCONTINENCE
The first step is to identify if the incontinence is secondary to diarrhea. Iif so, endoscopic mucosal evaluation, stool tests, and breath tests may be useful (12). Specific and complementary tests that can define the underlying mechanisms include anorectal manometry, anal endosonography and neurophysiological tests (12,18,19). An evidence based summary of commonly performed diagnostic tests is shown in Table 1.
Table 1.
Evidence-based summary of the utility of commonly performed diagnostic tests in fecal incontinence (modified from Ref. 1)
| Test | Clinical Utility | Evidence | Recommendation (Grade) | Comments | |
|---|---|---|---|---|---|
| Strengths | Weaknesses | ||||
| • Physiologic Tests | |||||
| ○ Anorectal Manometry | Quantifies sphincter pressures, rectal sensation & compliance & recto-anal reflexes | Lack of standardization | Good | B2 | Widely used. Facilitates diagnosis of incontinence and dyssynergic defecation |
| ○ Needle EMG | Quantifies Spike potentials and reinnervation pattern indicating neuropathy/myopathy | Invasive, painful, not widely available | Fair | B3 | Only used in research labs |
| ○ Surface EMG | Displays EMG activity and can provide information on normal or weak muscle tone | Inaccurate, Artifacts | Fair | B3 | Largely used for Biofeedback |
| ○ Pudendal Nerve Terminal Motor Latency | Measures latency of terminal portion of pudendal nerve, simple | Minimally invasive, low sensitivity, interobserver differences | Fair | B3 | Conflicting recommendations |
| ○ Translumbar and Transsacral Motor Evoked Potentials | Quantifies spino-anal and spino-rectal nerve conduction Minimally invasive, | Lack of Training & Controlled studies, Availability | Fair | B3 | Promising Noninvasive objective test, |
Anorectal Manometry & Sensory Testing
Anorectal manometry quantifies IAS and EAS function, rectal sensation, rectoanal reflexes and rectal compliance. Currently, several types of probes and pressure recording devices are available. Each system has distinct advantages and drawbacks (20). Although a water perfused probe has been traditionally used, increasingly, a solid-state probe with micro transducers or air filled miniaturized balloons are being used. Typically, after a run in period, several maneuvers are performed such as squeeze, party balloon inflation, attempted defecation, and intermittent rectal balloon distention to assess sphincter pressures, reflex changes, anal and rectal pressure changes and rectal sensation (12,19).
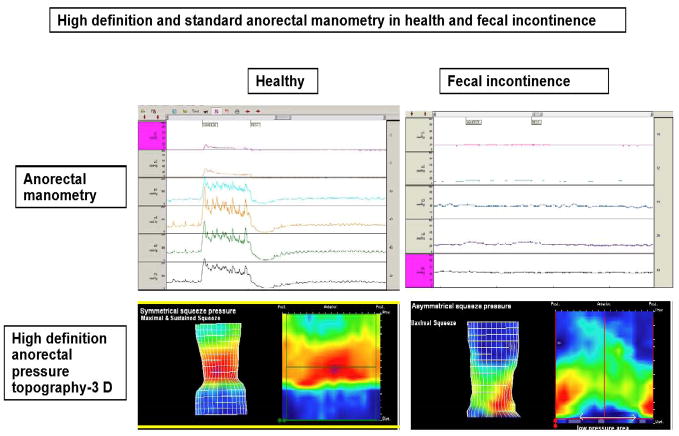
Recently, a novel solid-state probe with 12 circumferential sensors that provides a higher resolution (Sierra Scientific Instruments, Los Angeles, CA) has been introduced and this system correlates well with conventional manometry (20). This device uses novel pressure transduction technology (TactArray) that allows each sensor to detect pressure over a length of 2.5mm and in each of 12 radially dispersed sectors. The pressures can also be displayed as isobaric contour plots. The advantages are the detection of pressure changes over a longer length of rectum and anal canal increasing accuracy and detection of abnormalities. Also a high definition manometry system with 256 circumferentially arrayed sensors (21) that provides anal sphincter pressure profiles and topographic changes in 3-D is now available. This system further enhances our understanding [Figure 1].of anal pressure profiles, normal physiology, puborectalis function and sphincter defects, and may increase diagnostic yield
Fig 1.
High Definition Manometry and pressure topography during rest and voluntary squeeze. In the healthy subject (left), normal resting and normal increase in sphincter pressure is seen whereas in the incontinent subject the sphincter is weak during squeeze.
Patients with incontinence have low resting and/or low squeeze sphincter pressures (Figure 1) indicating weak IAS and EAS. The ability of EAS to contract reflexively can also be assessed during abrupt increases of intra-abdominal pressure such as when coughing. This reflex response is impaired in subjects with cauda equina lesions (19).
Rectal Sensory Testing
Rectal balloon distention with incremental volumes of air can be used for the assessment of both sensory responses and compliance. Incontinent patients may exhibit rectal hyposensitivity or hypersensitivity (19,22).
Rectal compliance is assessed by measuring the changes in intrarectal pressure during incremental rectal balloon distention (18). Rectal compliance is reduced in patients with colitis, low spinal cord lesions, and diabetics with incontinence but is increased in subjects with high spinal cord lesions (2).
Imaging the Anal Canal
Anal endosonography
It provides an assessment of the thickness and structural integrity of the EAS and IAS and can detect scarring, loss of muscle tissue and other local pathology (23). It is performed by using a 7–12 mHz rotating transducer with a focal length of 1 to 4 cm (24). More recently, 3-D ultrasound imaging has become available which provides better delineation of anal sphincters and puborectalis and surrounding structures (23).
After vaginal delivery, anal endosonography revealed occult sphincter injury in 35% of primipara women, and sphincter defects in 85% of women with third degree perineal tear compared with 33% without tears (25). Although endosonography can distinguish internal from external sphincter injury, it has low specificity for demonstrating the etiology of fecal incontinence (12). Because anal endosonography is widely available, less expensive and less painful than needle EMG, currently, this technique is preferred for the assessment of sphincter morphology (23).
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) provides superior imaging with better spatial resolution of the external anal sphincter (23,24). A major contribution of anal MRI has been the recognition of external sphincter atrophy, and sometimes without pudendal neuropathy. The addition of dynamic pelvic MRI by using fast imaging sequences or MRI colpocystography that involves rectal filling with ultrasound gel and having the patient evacuate while lying inside the magnet may each provide better delineation of pelvic anatomy (23). The use of an endo-anal coil significantly enhances the resolution and allows more precise definition of sphincter muscles. However, comparative studies with other technology, costs, and clinical utility have not been assessed.
Neurophysiologic testing of anorectal function
Electromyography (EMG) of the anal sphincter identifies sphincter injury as well as denervation-reinnervation potentials that indicate neuropathy (18,26). EMG can be performed using a needle electrode or surface electrode (26). Abnormal EMG activity, such as fibrillation potentials and high-frequency spontaneous discharges provide evidence of denervation. It occurs in patients with fecal incontinence following pudendal nerve injury or cauda equina syndrome (26,27). The pudendal nerve terminal motor latency (PNTML) measures the neuromuscular integrity between the terminal portion of the pudendal nerve and the anal sphincter. An injury to the pudendal nerve such as after forceps-assisted delivery leads to denervation of the anal sphincter muscle and muscle weakness. The AGA technical review did not recommend PNTML test (18), although experts suggest that PNTML may facilitate selection of patients prior to sphincter repair (28).
Motor Evoked Potentials
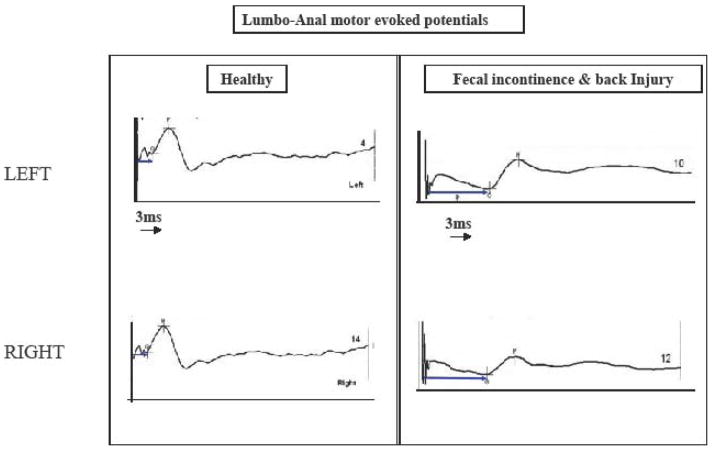
The integrity of the entire spino-anorectal pathways that control anorectal function can be assessed by magnetic stimulation and recording of motor evoked potentials (MEPs) (26). When a current is rapidly discharged through a conducting coil, a magnetic flux is produced around the coil, which causes stimulation of neural tissue. Electrical or magnetic stimulation of the lumbosacral nerve roots facilitates measurements of the conduction time within the cauda equina and diagnosis of sacral motor radiculopathy as a possible cause of fecal incontinence (29). A recent study showed that Translumbar MEP and Transsacral MEP of the rectum and anus provide better delineation of the peripheral neuromuscular injury in subjects with fecal incontinence (30) [Figure 2], and those with spinal cord injury, and is superior to PNTML (31).
Fig 2.
Neurophysiological changes following translumbar magnetic stimulation. In healthy subject (left), normal motor evoked potential with a short latency (arrow) can be seen whereas in the incontinent subject (right), the MEP onset is prolonged onset and amplitude is decreased indicating neuropathy.
Clinical Utility of tests for fecal incontinence
In one prospective study, history alone could detect an underlying cause in only 9 of 80 patients (11%) with incontinence whereas physiological tests revealed an abnormality in 44 patients (55%) (32). In another study, patients with incontinence had lower resting and squeeze sphincter pressures, a smaller rectal capacity and leaked earlier following saline infusion in the rectum (33). In a prospective study, anorectal manometry with sensory testing not only confirmed a clinical impression but also provided new diagnostic information that influenced management and outcome of patients with incontinence (12). Also, 80% of patients had more than one abnormality emphasizing the body’s’ ability to compensate for the loss of any one mechanism.(12). In another study, weak anal sphincters were found in 40 patients (71%) and altered rectal sensation or compliance in 42 patients (75%) (32), but tests alone were insufficient to predict whether an individual had incontinence.
An abnormal test result must be interpreted along with the patient’s symptoms and not merely by the manometric parameters. For the individual patient with incontinence, physiologic and morphologic testing can be very useful both for providing a diagnosis and for assessing objective improvement following a therapeutic intervention (12).
CONSTIPATION & DYSSYNERGIC DEFECATION
INTRODUCTION
Constipation is a polysymptomatic, multifactorial disorder that affects 15–20% of the population (34). It is more prevalent in women, elderly, non-caucasians and subjects with lower socio economic status (34). Constipation places a substantial burden on healthcare resources (35), and affects both quality of life and psychological function (36). Recently, significant advances have been made regarding the pathophysiology and diagnostic testing (22,23,37,38,39).
Primary constipation is due to altered colonic and anorectal neuromuscular function whereas secondary constipation results from poor fiber intake, drugs (eg opioids), behavioral, endocrine, metabolic, neurological and other disorders (40). At least three overlapping subtypes of primary constipation have been recognized (37,40). Slow transit constipation (STC) is characterized by impaired propulsion of stool and is due to dysfunction of colonic smooth muscle (myopathy) or its nerve innervation (neuropathy), or both or can be secondary to dyssynergic defecation (37). Evacuation disorders are characterized by difficulty or inability with stool expulsion. They include disorders of the anorectal function such as dyssynergic defecation (37), as well as structural disorders such as rectocele, descending perineum syndrome and rectal prolapse (37). Constipation predominant irritable bowel syndrome (IBS-C), is seen in patients in whom abdominal discomfort or pain is a prominent symptom together with symptoms of constipation (41). These patients may or may not have coexisting STC or evacuation disorder. This review will focus on the mechanisms and assessment of dyssynergic defecation.
Pathophysiology of Dyssynergic Defecation
Dyssynergia is an acquired behavioral disorder of defecation. In two thirds of subjects, dyssynergia is a consequence of faulty toilet habit, painful defecation, obstetric or back injury and brain-gut dysfunction (26,37). In the rest, the coordinated process of defecation was perhaps never learnt during childhood (37). Dyssynergic subjects demonstrate the inability to coordinate the abdominal, rectoanal and pelvic floor muscles during defecation (42,43). Additionally, 30–50% of subjects may exhibit rectal hyposensitivity (22,42).
Manometrically, at least four reproducible types of dyssynergia (37) have been described [Figure 3]. The recognition of these patterns allows the biofeedback therapist to expound patient-specific treatment programs, such as emphasizing the push effort (Type II) or improving pelvic relaxation (Type III). The diagnostic criteria for dyssynergic defecation are shown below (37).
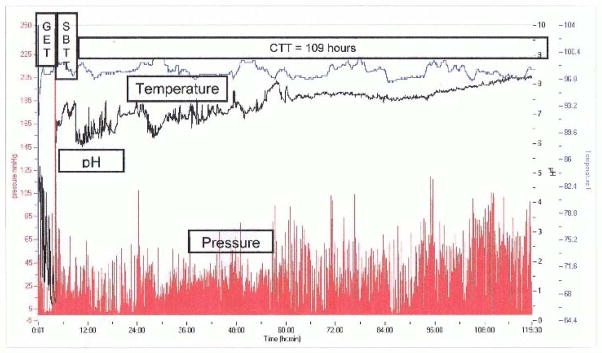
Fig 3.
Assessment of colonic, regional and whole gut transit with a wireless motility capsule in a subject with chronic constipation and dyssynergic defecation. The time is represented on the horizontal axis. The blue line represents temperature changes, the green line the pH changes and the red line the pressure changes. GET+ Gastric emptying time; SBTT= Small bowel transit time; CTT= Colonic transit time. The colonic transit is delayed in this subject normal CTT <59 hours) emphasizing the overlap between dyssynergic defecation and slow colonic transit.
Patients must satisfy the symptomatic diagnostic criteria for chronic constipation (Rome III) and
Patients must demonstrate a dyssynergic pattern of defecation (Types 1–4), during repeated attempts to defecate with manometry, imaging or electromyography. Dyssynergia is defined as a paradoxical increase in anal sphincter pressure (anal contraction) or less than 20% relaxation of the resting anal sphincter pressure or inadequate abdomino-rectal propulsive forces.
-
One or more of the following;
Inability to expel an artificial stool (50 ml water-filled balloon) within one minute.
Prolonged colonic transit time, i.e. greater than 5 markers (≥20% marker retention) on a plain abdominal x-ray taken 120 hours after ingestion of one sitzmark® capsule containing 24 radio opaque markers.
Inability to evacuate or ≥50% retention of barium during defecography.
Diagnostic Tests
It is important to obtain a detailed history with particular emphasis on stool habit and consistency. A recent study reaffirmed that stool consistency and not stool frequency correlated with transit time (44). Also a carefully performed DRE may reveal dyssynergia (sensitivity 77%) (45). Because a patient’s recall of stool habit is often inaccurate (46) and symptoms do not predict the underlying pathophysiology (37), diagnostic tests are required to facilitate management. The first step is to identify drug-induced, metabolic or colonic disorder, because constipation is caused by organic conditions and rarely colon cancer (40). The ACG Task Force does not routinely recommend tests in patients aged <50 years and in whom there are no alarm symptoms or signs (47). Alarm features include new onset of constipation, onset after age 50, bloody stools, weight loss, anemia or a family history of inflammatory bowel disease or colon cancer (47). For patients < 50 years without alarm features, empiric treatment without diagnostic testing is appropriate (47). Once an organic disorder has been excluded, most have a neuromuscular disorder of the colorectum. Physiologic testing should be considered in patients who are unresponsive to laxatives, and in those with an evacuation disorder (48).
Specific diagnostic tests for functional constipation
Constipation is a heterogeneous disorder and testing cannot mimic real-life physiology of stool transport and evacuation. Therefore, no single test adequately defines constipation, often more than one test is required (37,48). Colonic transit study, anorectal manometry, balloon expulsion test, and defecography are widely used and an evidence based summary of tests is shown in Table 3.
Colonic Transit Study
This test provides an objective measurement of the speed of stool movement through the colon. It is measured by one of three methods: radiopaque markers (39,49), colonic scintigraphy (50) or wireless motility capsule (SmartPill®) (39).
The radioopaque marker test is commonly performed by administering a single capsule containing 24 plastic markers (Sitzmarks®, Konsyl Pharmaceuticals, Fort Worth, Texas) on day 0 and by obtaining plain abdominal x-ray on day 5 (120 hours) later (39,49). Retention of ≥ 20% markers (≥6 markers) on day 5 (120 hrs) is considered abnormal and is indicative of STC. Because, 60% of patients with dyssynergic defecation demonstrate excessive retention of markers (37) that improves with therapy (37), a diagnosis of STC should be made only after excluding dyssynergia (37). A multiple capsule technique has also been used (51). The prevalence of STC based on colonic transit study varied from 38–80% largely due to methodological differences (48).
Recently, a novel, wireless motility capsule (SmartPill®, SmartPill Corporation, Buffalo, NY) that measures pressure, pH and temperature has been approved by FDA. This device provides a radiation-free method of measuring colonic, whole gut and regional (gastric emptying) transit, Figure 4, (39). Wireless capsule has good specificity, good device agreement with radioopaque markers, and provides a standardized method of assessment for subjects with and without STC (39) and has been validated (52).
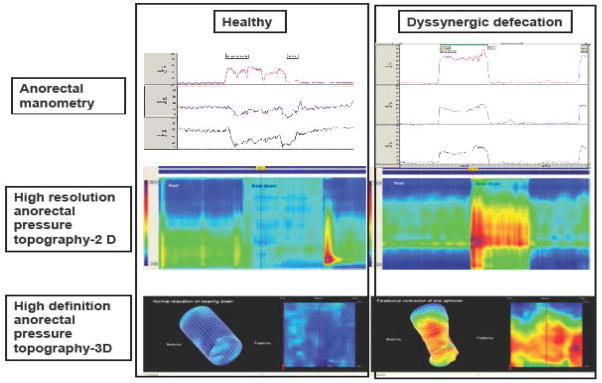
Fig 4.
Manometric and pressure topographic changes in a healthy individual (left) and a patient with dyssynergic defecation (right). In healthy subject, normal relaxation of anal sphincters can be seen both with manometry and topography whereas in the dyssynergic subject there is increase in rectal pressure with paradoxical increase in anal sphincter pressure seen both with manometry and topography.
Anorectal Manometry
Manometry quantifies the rectal and anal pressure changes during attempted defecation and reflex changes during balloon distention and thereby identifies dyssynergic defecation and Hirschsprung’s disease(19,37). When healthy subjects attempt defecation, they generate an adequate propulsive force (rise in intrarectal pressure) synchronized with relaxation of puborectalis and anal sphincter (decrease sphincter pressure) (37) [Figure 5]. Patients with dyssynergic defecation fail to perform this maneuver (42). The body position, whether sitting or lying down, the presence of stool-like sensation and the consistency of stool may each influence both defecation and the ability to expel stool (53). Hence, dyssynergic pattern alone is not diagnostic of dyssynergic defecation, and additional diagnostic features are recommended (37). Furthermore, sensory testing may reveal rectal hyposensitivity (22,37).
A systematic review revealed that dyssynergia is detected in approximately 50% of subjects with evacuation problems (37). High resolution manometry may provide better characterization of dyssynergia [Figure 5] (20,21). Thus, anorectal manometry is useful for diagnosis of dyssynergic defecation and altered rectal sensation and identifies subjects who could benefit with biofeedback therapy.
Balloon Expulsion Test
This provides bedside assessment of a subjects’ ability to expel an artificial stool. Most experts recommend a 50 ml water filled balloon. Normal subjects can expel this balloon within one minute (48). The prevalence of a positive test in favor of dyssynergia varies between 23–67% (48). One study suggested a specificity of 89%, negative predictive value of 97%, sensitivity of 88%, and positive predictive value of 67% (54). However, many dyssynergics can expel the balloon (37). Thus, although failure to expel a balloon suggests dyssynergia, a normal test does not exclude this possibility. Hence, this test should be interpreted along with other physiological tests. Also, patients with fecal seepage (12) and elderly subjects with fecal incontinence secondary to fecal impaction demonstrate an impaired balloon evacuation. A recent study showed a high prevalence of dyssynergia in nursing home residents with incontinence (55).
Rectal Barostat Test
An assessment of rectal sensation, tone and compliance using a highly compliant balloon that is placed in the rectum and connected to a computerized pressure distending device (barostat) can be useful for detecting rectal hyposensitivity (22) and for identifying patients with normal, impaired or hyper-compliant rectum, and detection of megarectum. Likewise, rectal barostat studies can reveal rectal hypersensitivity in up to 50% of patients with IBS-C (1,22).
Defecography
This test provides morphological information and uses fluoroscopy (23). Approximately 150 ml of contrast is placed into the rectum and the subject is asked to squeeze, cough or expel the contrast. The most common findings are; poor activation of levator muscles, prolonged retention or inability to expel the barium, absence of a stripping wave in the rectum, mucosal intussusception and/or rectocele (18,23). The prevalence of normal defecography varied between 10 to 75% (18). Although defecography revealed abnormalities in 77% of subjects, there was no relationship between symptoms and abnormalities (28,23). Among ten studies, abnormalities were reported in 25–90% and dyssynergia in 13–37% (48).
Disadvantages of defecography include radiation exposure, embarrassment, inter observer bias and inconsistent methodology. Hence, defecography is recommended as an adjunct to clinical and manometric assessment (18,48).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) and dynamic pelvic MRI- “MR defecography” can be useful for assessment of anorectal disorders (23,24,56). In fact, this is the only imaging modality that can simultaneously evaluate global pelvic floor anatomy and dynamic motion (23) [Figure 6]. The free selection of imaging planes, lack of radiation exposure, good temporal resolution, and excellent soft-tissue contrast are advantages. Dynamic pelvic MRI in the sitting position provides a more physiological approach than supine position (57).
Dynamic MRI is useful, because it can differentiate between mucosal and full-thickness rectal prolapse (56). In dyssynergic patients, dynamic MRI revealed that the anorectal angle became more acute, confirming paradoxical contraction of puborectalis (24). Recently, in a controlled study, during rectal evacuation, the degree of perineal descent was decreased in 35%, normal in 44%, and increased in 21% of constipated patients (56). Increased perineal descent was associated with a hypertensive anal sphincter, a normal rectal balloon expulsion test, and a rectocele. Limitations of MRI defecography include its high cost, lack of standardization and availability.
CONCLUSIONS
A practical knowledge of pelvic floor structure and function will enable the gastroenterologist to seek appropriate clues for etiology. Symptom diaries and digital rectal examination can provide useful assessment of sphincter pressure, presence of dyssynergia and fecal impaction. Anorectal manometry with rectal sensory testing is the preferred method for defining the functional weakness of the anal sphincter and for diagnosis of dyssynergia and abnormal rectal sensation. Newer tests such as high definition manometry may provide better understanding through 3-D and topographic mapping. Evolving tools such as motor evoked potentials may provide comprehensive neurophysiological information. Anal endosonography can define structural defects with 3-D ultrasound providing superior resolution. Defecography is useful in patients with suspected rectal prolapse or poor rectal evacuation. Balloon expulsion test can confirm impaired evacuation but by itself is not diagnostic. These tests should be performed either to confirm a clinical suspicion or to elucidate a cause for refractory bowel symptoms. There is good evidence to support the use of these physiological tests and to define the underlying pathophysiology in subjects with fecal incontinence and constipation and to guide treatment.
Supplementary Material
Anorectal manometry showing a normal pattern of defecation in the center panel, which consists of a good pushing force (increase in intra rectal pressure) coordinated with relaxation of anal sphincter. Subjects with dyssynergic defecation will exhibit one of four abnormal patterns of defecation. In type I dyssynergia, the subject can generate an adequate propulsive force (rise in intra rectal pressure ≥40 mmHg) along with paradoxical increase in anal sphincter pressure. In type II dyssynergia, the subject is unable to generate an adequate propulsive force together with paradoxical anal contraction. In type III dyssynergia, the subject can generate an adequate propulsive force along with an absent relaxation (a flat line) or incomplete (≤20%) relaxation of resting anal sphincter pressure. In type IV dyssynergia, the subject is unable to generate an adequate propulsive force together with absent or incomplete relaxation of anal sphincter pressure.
Table 2.
Evidence-based summary of the utility of the diagnostic tests for chronic constipation (modified from Remes-Troche and Rao, (Ref. 1)
| Test | Clinical Utility | Evidence | Recommendation (Grade) | Comment | |
|---|---|---|---|---|---|
| Strength | Weakness | ||||
| Blood tests (thyroid, calcium, glucose, electrolytes) | Rule out metabolic disorder | Not cost-effective | No evidence | C | Not recommended routinely without alarm features Not |
| Imaging tests Plain abdominal X-Ray |
Identify excessive amount of stool in the colon, simple, inexpensive, widely available | Lack of standardization and controlled studies | Poor | C | None |
| Barium enema | Identify megacolon, megarectum, stenosis, diverticulosis, masses | Lack of standardization, Radiation exposure. Lack of controlled studies |
Poor | C | Not recommended for routine without alarm features evaluation |
| Defecography | Identify dyssynergia, rectocele, prolapse, excessive descent, megarectum, Hirschsprung disease | Radiation exposure, embarrassment, interobserver bias, inconsistent methodology | Fair | B3 | Used as adjunct |
| Anorectal ultrasound | Visualization of the internal anal | Interobserver bias, availability. | Poor | C | Experimental |
| MRI | Simultaneously evaluate global pelvic floor anatomy, sphincter morphology and dynamic motion | Expensive, lack of standardization, availability | Fair | B3 | Used as adjunct to anorectal manometry |
| Flexible sigmoidoscopy and colonoscopy | Visualization of mucosal disease | Invasive, risks of procedure and sedation | Poor | C | Lack of prospective study regarding efficacy |
| Physiologic testing | |||||
| Colonic transit with radiopaque markers | Evaluate colon transit, inexpensive and widely available | Inconsistent methodology | Good | B2 | Useful to identify slow transit constipation |
| Colonic transit with scintigraphy | Evaluate slow, normal or rapid colonic transit. | Expensive, time consuming, availability, lack of standardization | Good | B2 | Facilitates classification of pathopysiological subtypes |
| Wireless Motility Capsule | Standardized evaluation of slow, normal or rapid colonic and upper gastrointestinal transit No Radiation, Validated technique | Availability | Excellent | A1 | Reliably identifies slow transit constipation and upper gut transit abnormalities |
| Anorectal Manometry | Identify dyssynergic defecation, rectal hyposensitivity, & hypersensitivity, impaired compliance, | Lack of standardization | Good | B2 | Facilitates diagnoses of dyssynergic defecation, Rectal sensory problems and Hirschsprung’s disease |
| Balloon expulsion test (BET) | Bedside assessment of dyssynergic defecation | Lack of standardization | Good | B2 | Normal BET does not exclude dyssynergia. |
| Colonic manometry | Identify colonic myopathy, neuropathy Facilitates selection of patients for surgery | Invasive, not widely available, lack of standardization | Fair | B3 | Adjunct to colorectal function tests |
Grade A1: Excellent evidence in favor of the test based on high specificity, sensitivity, accuracy and positive predictive values
Grade B2: Good evidence in favor of the test with some evidence on specificity, sensitivity, accuracy, and predictive values
Grade B3: Fair evidence in favor of the test with some evidence on specificity, sensitivity, accuracy, and predictive values
Grade C: Poor evidence in favor of the test with some evidence on specificity, sensitivity, accuracy, and predictive values
Acknowledgments
Grant Support: This work was supported in part by NIH grant RO1 DK 57100-05
Footnotes
Disclosures: Dr. Rao serves on the Advisory Board for SmartPill® Corporation and has received research support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Remes-Troche JM, Rao SS. Defecation disorders: neuromuscular aspects and treatment. Curr Gastroenterol Rep. 2006;8:291–299. doi: 10.1007/s11894-006-0049-x. [DOI] [PubMed] [Google Scholar]
- 2.Rao SSC. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–S22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein MM, Pretorius DH, Jung SA, et al. Transperineal three-dimensional Ultrasound imaging for detection of anatomic defects in the anal sphincter complex. Muscles Clin Gastroeneterol Hepatol. 2009;7:205–211. doi: 10.1016/j.cgh.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azpiroz F, Fernandez-Fraga X, Merletti R, et al. The puborectalis muscle. Neurogastroenter Motil. 2005;17(Suppl 1):68–72. doi: 10.1111/j.1365-2982.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 6.De Ocampo S, Remes-Troche JM, Miller M, et al. Rectoanal sensori-motor response in humans during rectal distension. Dis Colon Rectum. 2007;50:1639–1646. doi: 10.1007/s10350-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 7.Remes-Troche JM, De Ocampo S, Miller M, et al. Investigation of sensori-motor, recto anal inhibitory and contractile reflexes in rectal hyposensitivity. Dis Colon Rectum. (In Press) [Google Scholar]
- 8.Zagorodnyuk VP, Lynn P, Costa M, et al. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–7. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharucha AE, Fletcher JG, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nygaard I, Barber MD, Burgio KL. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SSC. Practice Guidelines: Diagnosis and Management of Fecal Incontinence. Am J Gastroenterol. 2004;99:1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 13.Tantiphlachiva K, Chahal P, Feyen B, et al. Trainee versus Expert assessment of Digital Rectal Examination for Anorectal Dysfunction: Does experience matter? A Prospective Study Am J Gastroenterol. 2009;104(3):S498. [Google Scholar]
- 14.Hill J, Corson RJ, Brandon H, et al. History and examination in the assessment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 1994;37:473–477. doi: 10.1007/BF02076194. [DOI] [PubMed] [Google Scholar]
- 15.Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SGM. Investigation of anorectal function. Br J Surg. 1988;75:53–55. doi: 10.1002/bjs.1800750119. [DOI] [PubMed] [Google Scholar]
- 16.Rockford TH, Church JM, Fleshman JW, et al. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. [DOI] [PubMed] [Google Scholar]
- 17.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on Anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 19.Rao SSC, Azpiroz F, Diamant N, et al. Minimum Standards of Anorectal Manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water perfused manometry. Am J Gastroenterol. 2007;102:850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 21.Tantiphlachiva K, Attaluri A, Rao SSC. Is high-definition manometry a comprehensive test of anal sphincter function: Comparative study with manometry and ultrasound Neurogastro. Mot. 2008;20:S28. [Google Scholar]
- 22.Scott SM, Gladman MA. Manometric, Sensorimotor, and Neurophysiologic Evaluation of Anorectal Function. Gastroenterol Clin N Am. 2008;37:511–538. doi: 10.1016/j.gtc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Savoye-Collet C, Koning E, Dacher J. Radiologic Evaluation of Pelvic Floor Disorders. Gastroenterol Clin N Am. 2008;37:553–567. doi: 10.1016/j.gtc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher JG, Busse RF, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 25.Sultan AH, Kamm MA, Hudson CN, Bartram CI. Third degree obstetric anal sphincter tears: risk factors and outcome of primary repair. Br Med J. 1994;308:887–891. doi: 10.1136/bmj.308.6933.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remes-Troche J, Rao SSC. Neurophysiological testing in anorectal disorders. Gastroenterol Hepatol. 2008;2:323–35. doi: 10.1586/17474124.2.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilad R, Giladi N, Korczyn AD, et al. Quantitative anal sphincter EMG in multisystem atrophy and 100 controls. J Neurol Neurosurg Psychiatry. 1997;71:596–599. doi: 10.1136/jnnp.71.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothholtz NA, Wexner SD. Surgical treatment of constipation and fecal incontinence. In: Rao SSC, editor. Disorders of Anorectum. Vol. 30. Gastroenterol Clin North Am. W.B. Saunders; 2001. pp. 131–166. [DOI] [PubMed] [Google Scholar]
- 29.Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo0sacral stimulation: normative data. J Neurol Sci. 1997;149:69–72. doi: 10.1016/s0022-510x(97)05388-4. [DOI] [PubMed] [Google Scholar]
- 30.Rao SSC, Tantiphlachiva K, Attaluri A, et al. Translumbar and Transsacral Magnetic Stimulation- A novel test of assessing anorectal neuropathy in fecal incontinence. Gastroenterology. 2008;134:A-278. [Google Scholar]
- 31.Tantiphlachiva K, Remes-Troche J, Attaluri A, et al. Evaluation of spino-anorectal pathways in spinal cord injury with bowel dysfunction using magnetic stimulation: a novel and noninvasive test. Gastroenterology. 2008;134:A-274. [Google Scholar]
- 32.Wexner SD, Jorge JM. Colorectal physiological tests: use or abuse of technology? Br Jr Surg. 1994;160:167–174. [PubMed] [Google Scholar]
- 33.Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SGM. Anorectal function investigations in incontinent and continent patients: Difference and Discriminatory Value. Dis Colon Rectum. 1990;33:479–486. doi: 10.1007/BF02052142. [DOI] [PubMed] [Google Scholar]
- 34.Higgins PD, Johanson JF. Epidemiology of Constipation in North America: A Systematic Review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 35.Singh G. Adults with Constipation Account for Significant Use of Healthcare Resources and Costs of Care. Clin Gastroenterol Hepatol. 2007;5:1053–1058. doi: 10.1016/j.cgh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Rao SSC, Kinkade K, Schulze K, Nygaard I, Stumbo P, Zimmerman B. Do Psychological Profiles and Quality of Life (QOL) Differ in Patients with Dyssynergia and Slow Transit Constipation? J Psychoson Med. 2007;63:441–449. doi: 10.1016/j.jpsychores.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Rao SSC. Dyssynergic Defecation and Biofeedback Therapy. Gastroenterol Clin N Am. 2008;37:569–586. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camilleri M, Bharucha AE, di Lorenzo C. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–82. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 39.Rao SSC, Kuo B, McCallum R. Investigation of Colonic and Whole Gut Transit with Wireless Motility Capsule and Radioopaque Markers in Constipation. Clinic Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 41.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 42.Rao SSC, Welcher KD, Leistikow JS. Obstructive Defecation: A Failure of Rectoanal Coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 43.Bharucha AE, Croak AJ, Gebhart JB, Berglund LJ, Seide BM, Zinsmeister AR, An KN. Comparison of rectoanal axial forces in health and functional defecatory disorders. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1164–1169. doi: 10.1152/ajpgi.00487.2005. [DOI] [PubMed] [Google Scholar]
- 44.Saad RJ, Rao SS, Koch KL, et al. Do Stool Form and Frequency Correlate With Whole-Gut and Colonic Transit? Results From a Multicenter Study in Constipated Individuals and Healthy Controls. Am J Gastroenterol. 2009 Nov 3; doi: 10.1038/ajg.2009.612. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Rao P, Tantiphlachiva K, Attaluri A, Rao SSC. Is digital rectal examination useful in patients with dyssynergia? Clinic Gastroenterol Hepatol. 2010 doi: 10.1016/j.cgh.2010.06.031. (In Press) [DOI] [PubMed] [Google Scholar]
- 46.Ashraf W, Park F, Quigley EMM, Lof J. An Examination of the Reliability of Reported Stool Frequency in the Diagnosis of Idiopathic Constipation. Am J Gastroenterol. 1996;91:26–23. [PubMed] [Google Scholar]
- 47.Brandt LJ, Schoenfeld P, Prather CM, Quigley EMM, Schiller LR, Talley NJ. Evidence-based position statement on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100:S1–22. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 48.Rao SSC, Ozturk R, Laine L. Clinical utility of diagnostic test for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:101. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 49.Evans RC, Kamm MA, Hinton JM, Lennard-Jones JE. The normal range and a simple diagram of recording whole gut transit time. Int J Colorectal Dis. 1992;7:15–17. doi: 10.1007/BF01647654. [DOI] [PubMed] [Google Scholar]
- 50.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–15. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 51.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 52.Camilleri M, Thorne N, Ringel Y, et al. Wireless pH motility capsule for colonic transit: prospective comparison with radio opaque markers for chronic constipation. Neurogastro Mot. 2010 doi: 10.1111/j.1365-2982.2010.01517.x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao SSC, Kavlock R, Rao SSC. Influence of Body Position and Stool Characteristics on Defecation in Humans. Am J Gastroenterol. 2006;101:2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 54.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 55.Leung F, Beard M, Grbic V, Habermann R, Rao SSC, Schnelle J. Dyssynergia – Key Pathophysiologic Mechanism for Fecal Incontinence (FI) in Nursing Home Residents. Am J Gastroenterol. 2007;386:S257. [Google Scholar]
- 56.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223:501–8. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anorectal manometry showing a normal pattern of defecation in the center panel, which consists of a good pushing force (increase in intra rectal pressure) coordinated with relaxation of anal sphincter. Subjects with dyssynergic defecation will exhibit one of four abnormal patterns of defecation. In type I dyssynergia, the subject can generate an adequate propulsive force (rise in intra rectal pressure ≥40 mmHg) along with paradoxical increase in anal sphincter pressure. In type II dyssynergia, the subject is unable to generate an adequate propulsive force together with paradoxical anal contraction. In type III dyssynergia, the subject can generate an adequate propulsive force along with an absent relaxation (a flat line) or incomplete (≤20%) relaxation of resting anal sphincter pressure. In type IV dyssynergia, the subject is unable to generate an adequate propulsive force together with absent or incomplete relaxation of anal sphincter pressure.