Abstract
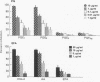
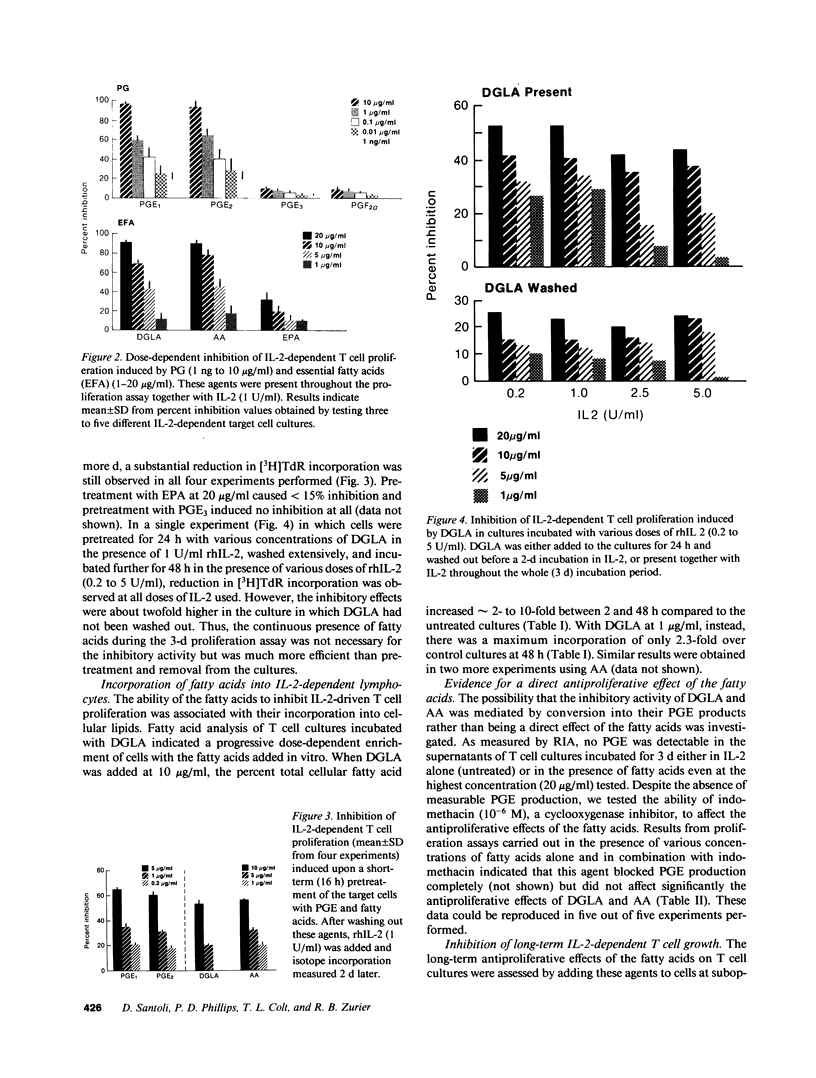
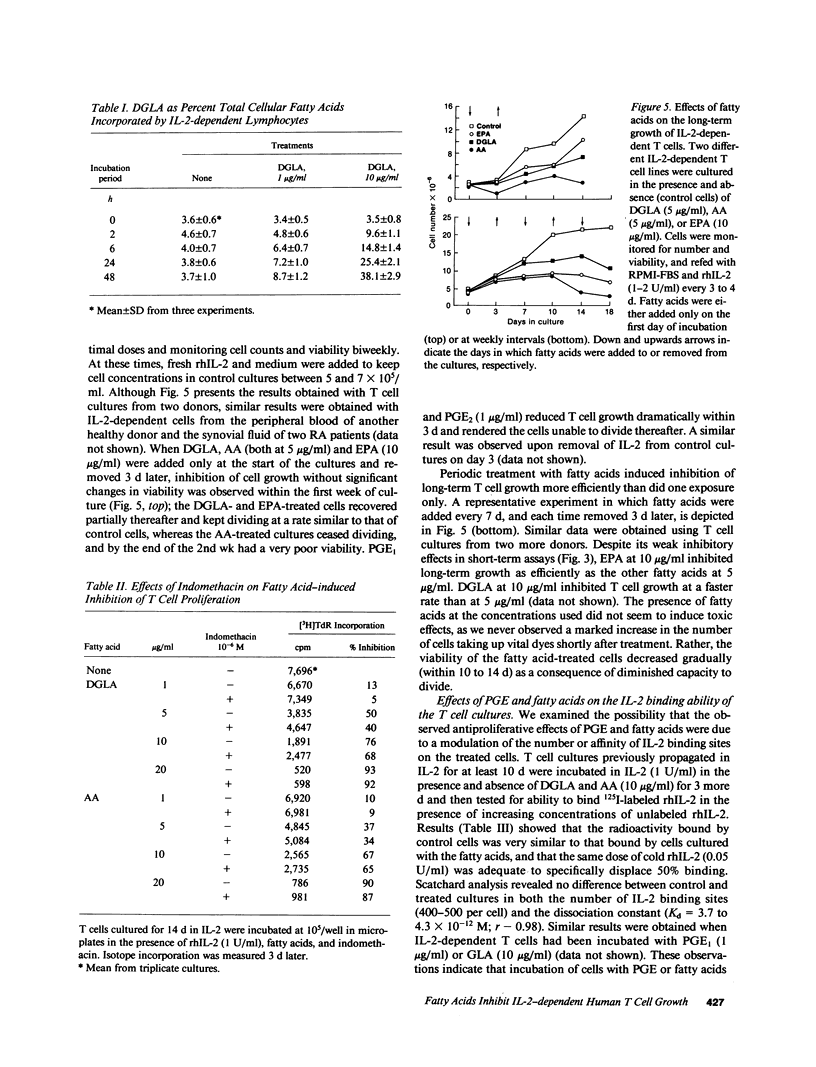
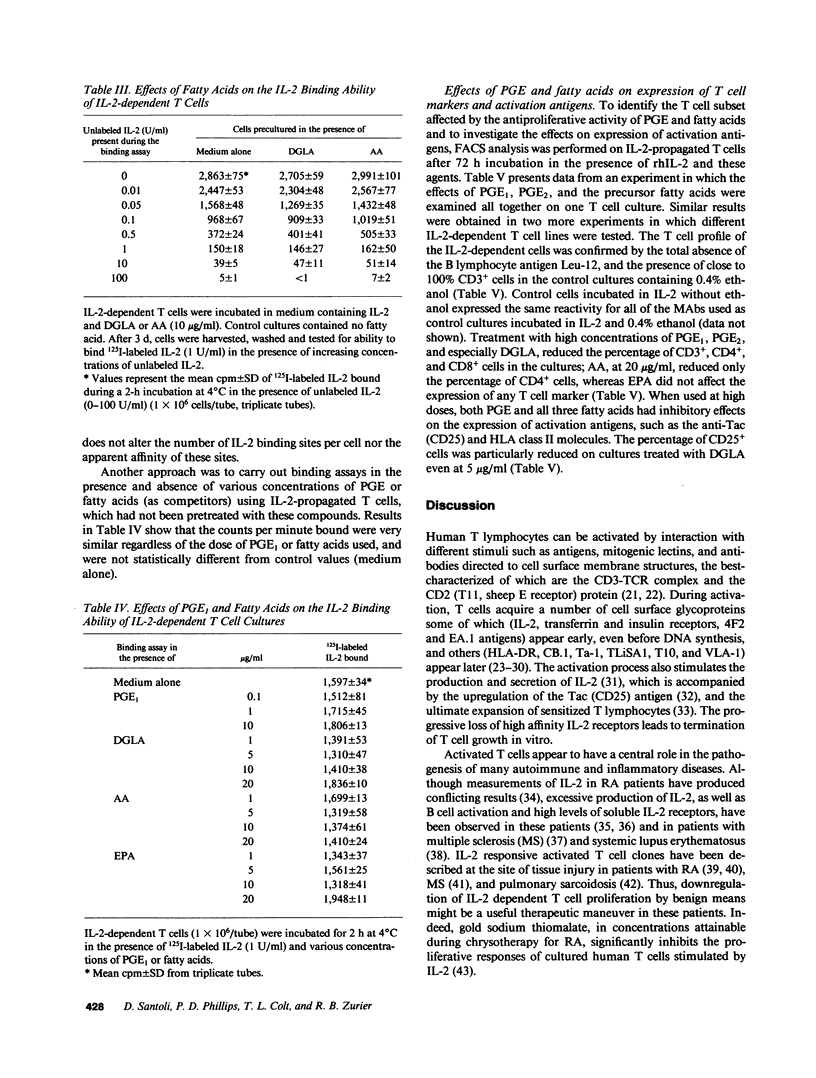
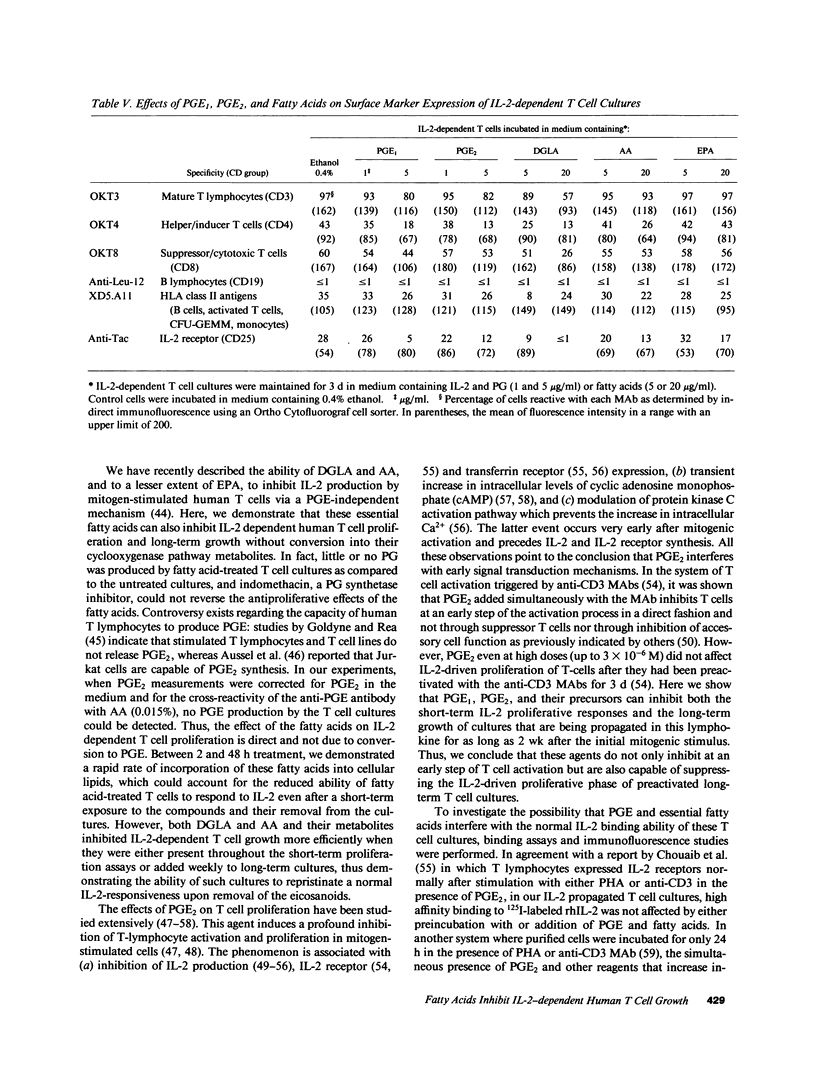
PGE represent oxygenation products of polyunsaturated essential fatty acids and are important regulators of cell-mediated immune responses. Because oils enriched in such fatty acids reduce inflammation and tissue injury in vivo, we examined the effects of these PGE precursors on IL-2-driven growth of human T lymphocytes. Dihomogamma linoleic acid (DGLA), AA, and their metabolites (PGE1 and PGE2, respectively) strongly inhibited short- and long-term growth of IL-2-dependent T cell cultures; EPA was much less inhibitory and its product, PGE3, failed to suppress IL-2 responses. Short-term pretreatment of the cells with DGLA or AA and removal of the fatty acids before the proliferation assay resulted in a smaller reduction in [3H]TdR incorporation. PGE and fatty acids did not alter the number of high affinity IL-2 binding sites on the T cell cultures but reduced the percentage of cells expressing CD25 and HLA class II molecules. No PGE was detected in supernatants from the fatty acid-treated cultures. Moreover, indomethacin, a cyclooxygenase inhibitor, did not reverse the antiproliferative effects of the fatty acids. Together, these findings indicate that fatty acids can inhibit IL-2-driven T cell growth via a PGE-independent mechanism and might be relevant to inflammatory diseases associated with persistent T cell activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aussel C., Mary D., Fehlmann M. Prostaglandin synthesis in human T cells: its partial inhibition by lectins and anti-CD3 antibodies as a possible step in T cell activation. J Immunol. 1987 May 15;138(10):3094–3099. [PubMed] [Google Scholar]
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. E., Fahey J. V., Munck A. Prostaglandin inhibition of T-cell proliferation is mediated at two levels. Cell Immunol. 1981 Jun;61(1):52–61. doi: 10.1016/0008-8749(81)90353-1. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Farrar W. L. Potentiation of lymphokine-activated killer cell differentiation and lymphocyte proliferation by stimulation of protein kinase C or inhibition of adenylate cyclase. J Immunol. 1988 Jan 1;140(1):208–214. [PubMed] [Google Scholar]
- Belch J. J., Ansell D., Madhok R., O'Dowd A., Sturrock R. D. Effects of altering dietary essential fatty acids on requirements for non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a double blind placebo controlled study. Ann Rheum Dis. 1988 Feb;47(2):96–104. doi: 10.1136/ard.47.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittiner S. B., Tucker W. F., Cartwright I., Bleehen S. S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988 Feb 20;1(8582):378–380. doi: 10.1016/s0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Buchan G., Barrett K., Fujita T., Taniguchi T., Maini R., Feldmann M. Detection of activated T cell products in the rheumatoid joint using cDNA probes to Interleukin-2 (IL-2) IL-2 receptor and IFN-gamma. Clin Exp Immunol. 1988 Feb;71(2):295–301. [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Chouaib S., Robb R. J., Welte K., Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987 Aug;80(2):333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Mertelsmann R., Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172–1179. [PubMed] [Google Scholar]
- Cohen P., Broekman M. J., Verkley A., Lisman J. W., Derksen A. Quantification of human platelet inositides and the influence of ionic environment on their incorporation of orthophosphate-32P. J Clin Invest. 1971 Apr;50(4):762–772. doi: 10.1172/JCI106547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droller M. J., Lindgren J. A., Claessen H. E., Perlmann P. Production of prostaglandin E2 by bladder tumor cells in tissue culture and a possible mechanism of lymphocyte inhibition. Cell Immunol. 1979 Oct;47(2):261–273. doi: 10.1016/0008-8749(79)90336-8. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Kelley V. E., Georgilis K., Lonnemann G., van der Meer J. W., Cannon J. G., Rogers T. S., Klempner M. S., Weber P. C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Evans S. W., Rapp U. R., Cleveland J. L. Effects of anti-proliferative cyclic AMP on interleukin 2-stimulated gene expression. J Immunol. 1987 Sep 15;139(6):2075–2080. [PubMed] [Google Scholar]
- Fischer A., Le Deist F., Durandy A., Griscelli C. Separation of a population of human T lymphocytes that bind prostaglandin E2 and exert a suppressor activity. J Immunol. 1985 Feb;134(2):815–819. [PubMed] [Google Scholar]
- Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987 Mar 1;138(5):1346–1350. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Acuto O., Poole C., Palley L., Daley J. F., Schlossman S. F., Reinherz E. L. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984 Sep;133(3):1250–1256. [PubMed] [Google Scholar]
- Fox D. A., Schlossman S. F., Reinherz E. L. Regulation of the alternative pathway of T cell activation by anti-T3 monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1945–1950. [PubMed] [Google Scholar]
- Goldyne M. E., Rea L. Stimulated T cell and natural killer (NK) cell lines fail to synthesize leukotriene B4. Prostaglandins. 1987 Dec;34(6):783–795. doi: 10.1016/0090-6980(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. P., Parker C. W. Effects of arachidonic acid and other unsaturated fatty acids on mitogenesis in human lymphocytes. J Immunol. 1979 Apr;122(4):1556–1562. [PubMed] [Google Scholar]
- Konishi K., Moller D. R., Saltini C., Kirby M., Crystal R. G. Spontaneous expression of the interleukin 2 receptor gene and presence of functional interleukin 2 receptors on T lymphocytes in the blood of individuals with active pulmonary sarcoidosis. J Clin Invest. 1988 Sep;82(3):775–781. doi: 10.1172/JCI113678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen Y. T., Bergroth V., Kinnunen E., Nordström D., Kouri T. Activated T lymphocytes in patients with multiple sclerosis in clinical remission. J Neurol Sci. 1987 Nov;81(2-3):133–139. doi: 10.1016/0022-510x(87)90090-6. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Jubiz W., Michalek A., Rynes R. I., Bartholomew L. E., Bigaouette J., Timchalk M., Beeler D., Lininger L. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987 Apr;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- Levine L. Levels of 13,14-dihydro-15-keto-PGE2 in some biological fluids as measured by radioimmunoassay. Prostaglandins. 1977;14(6):1125–1139. doi: 10.1016/0090-6980(77)90290-8. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Zubler R. H., Nabholz M., MacDonald H. R. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature. 1985 Jun 20;315(6021):669–672. doi: 10.1038/315669a0. [DOI] [PubMed] [Google Scholar]
- Maca R. D. The combined effect of prostaglandin E2 and interleukin 1 on interleukin 2 production and lymphocyte proliferation. Int J Immunopharmacol. 1987;9(5):611–618. doi: 10.1016/0192-0561(87)90128-7. [DOI] [PubMed] [Google Scholar]
- Mary D., Aussel C., Ferrua B., Fehlmann M. Regulation of interleukin 2 synthesis by cAMP in human T cells. J Immunol. 1987 Aug 15;139(4):1179–1184. [PubMed] [Google Scholar]
- Merrill J. E., Mohlstrom C., Uittenbogaart C., Kermaniarab V., Ellison G. W., Myers L. W. Response to and production of interleukin 2 by peripheral blood and cerebrospinal fluid lymphocytes of patients with multiple sclerosis. J Immunol. 1984 Oct;133(4):1931–1937. [PubMed] [Google Scholar]
- Mertin J., Stackpoole A., Shumway S. Nutrition and immunity: the immunoregulatory effect of n-6 essential fatty acids is mediated through prostaglandin E. Int Arch Allergy Appl Immunol. 1985;77(4):390–395. doi: 10.1159/000233814. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Lopez-Botet M., Moretta L. Involvement of T44 molecules in an antigen-independent pathway of T cell activation. Analysis of the correlations to the T cell antigen-receptor complex. J Exp Med. 1985 Sep 1;162(3):823–838. doi: 10.1084/jem.162.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Phillips P. D., Pignolo R. J., Cristofalo V. J. Insulin-like growth factor-I: specific binding to high and low affinity sites and mitogenic action throughout the life span of WI-38 cells. J Cell Physiol. 1987 Oct;133(1):135–143. doi: 10.1002/jcp.1041330117. [DOI] [PubMed] [Google Scholar]
- Plaut M. The role of cyclic AMP in modulating cytotoxic T lymphocytes. I. In-vivo-generated cytotoxic lymphocytes, but not in vitro-generated cytotoxic lymphocytes, are inhibited by cyclic AMP-active agents. J Immunol. 1979 Aug;123(2):692–701. [PubMed] [Google Scholar]
- Quill H., Gaur A., Phipps R. P. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J Immunol. 1989 Feb 1;142(3):813–818. [PubMed] [Google Scholar]
- Redondo J. M., Fresno M., López-Rivas A. Inhibition of interleukin 2-induced proliferation of cloned murine T cells by glucocorticoids. Possible involvement of an inhibitory protein. Eur J Immunol. 1988 Oct;18(10):1555–1559. doi: 10.1002/eji.1830181013. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Rincón M., Tugores A., López-Rivas A., Silva A., Alonso M., De Landázuri M. O., López-Botet M. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988 Nov;18(11):1791–1796. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Klein M. Target-effector interaction in the natural killer cell system. IV. Modulation by cyclic nucleotides. J Immunol. 1979 Dec;123(6):2785–2790. [PubMed] [Google Scholar]
- Rogers T. J., DeHaven J. I., Donnelly R. P., Lamb B. Suppression of B-cell and T-cell responses by the prostaglandin-induced T-cell-derived suppressor (PITS). II. Resolution of multiple PITS beta factors. Cell Immunol. 1984 Sep;87(2):703–707. doi: 10.1016/0008-8749(84)90039-x. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Santoli D., Defreitas E. C., Sandberg-Wollheim M., Francis M. K., Koprowski H. Phenotypic and functional characterization of T cell clones derived from the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 1984 May;132(5):2386–2392. [PubMed] [Google Scholar]
- Santoli D., Zurier R. B. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol. 1989 Aug 15;143(4):1303–1309. [PubMed] [Google Scholar]
- Seelig J., Waespe-Sarcevic N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry. 1978 Aug 8;17(16):3310–3315. doi: 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Kaduce T. L., Hoak J. C., Fry G. L. Utilization of arachidonic and linoleic acids by cultured human endothelial cells. J Clin Invest. 1981 Oct;68(4):1003–1011. doi: 10.1172/JCI110322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Kennedy M. S., Goldyne M. E. Prostaglandin E modulation of the mitogenic response of human T cells. Differential response of T-cell subpopulations. J Clin Invest. 1979 Nov;64(5):1188–1203. doi: 10.1172/JCI109572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons J. A., Wood N. C., Di Giovine F. S., Duff G. W. Soluble IL-2 receptor in rheumatoid arthritis. Correlation with disease activity, IL-1 and IL-2 inhibition. J Immunol. 1988 Oct 15;141(8):2612–2618. [PubMed] [Google Scholar]
- Tate G. A., Mandell B. F., Karmali R. A., Laposata M., Baker D. G., Schumacher H. R., Jr, Zurier R. B. Suppression of monosodium urate crystal-induced acute inflammation by diets enriched with gamma-linolenic acid and eicosapentaenoic acid. Arthritis Rheum. 1988 Dec;31(12):1543–1551. doi: 10.1002/art.1780311211. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Goldman C. K., Bongiovanni K. F., Chan W. C., Winton E. F., Yagita M., Grimm E. A., Waldmann T. A. The p75 peptide is the receptor for interleukin 2 expressed on large granular lymphocytes and is responsible for the interleukin 2 activation of these cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5394–5398. doi: 10.1073/pnas.84.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J., Wolf B. A., Lefkowith J. B., Stump W. T., McDaniel M. L. Glucose-induced phospholipid hydrolysis in isolated pancreatic islets: quantitative effects on the phospholipid content of arachidonate and other fatty acids. Biochim Biophys Acta. 1986 Dec 5;879(3):399–409. doi: 10.1016/0005-2760(86)90232-8. [DOI] [PubMed] [Google Scholar]
- Vercammen C., Ceuppens J. L. Prostaglandin E2 inhibits human T-cell proliferation after crosslinking of the CD3-Ti complex by directly affecting T cells at an early step of the activation process. Cell Immunol. 1987 Jan;104(1):24–36. doi: 10.1016/0008-8749(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington R. J. Interleukin-2 abnormalities in systemic lupus erythematosus and rheumatoid arthritis. A role for overproduction of interleukin-2 in human autoimmunity? J Rheumatol. 1988 Apr;15(4):616–620. [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Brelsford W. G. Soluble interleukin-2 receptors in systemic lupus erythematosus. Arthritis Rheum. 1988 Jun;31(6):729–735. doi: 10.1002/art.1780310605. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Hall V. C. Inhibition of in vitro proliferative response of cultured T lymphocytes to interleukin-2 by gold sodium thiomalate. Arthritis Rheum. 1988 Feb;31(2):176–181. doi: 10.1002/art.1780310204. [DOI] [PubMed] [Google Scholar]