Abstract
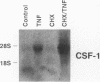
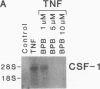
The effects of tumor necrosis factor (TNF) on the regulation of macrophage-specific colony stimulating factor (CSF-1) gene expression have been studied in HL-60 cells during monocytic differentiation. CSF-1 transcripts were undetectable in uninduced HL-60 cells, reached maximal levels by 3 h of exposure to TNF, and returned to that of control cells by 24 h. Transcriptional run-on analysis demonstrated that exposure to TNF stimulated the rate of CSF-1 gene transcription by 6.4-fold. The combination of a protein synthesis inhibitor, cycloheximide, and TNF increased levels of CSF-1 mRNA compared with treatment by TNF alone. We also studied the signal transduction mechanisms responsible for regulating TNF-induced CSF-1 mRNA levels. Both 4-bromophenacyl bromide and quinacrine, inhibitors of phospholipase A2 activity, blocked TNF-induced increases in CSF-1 transcripts in a concentration-dependent manner, while caffeic acid and nordihydroguaiaretic acid, inhibitors of the 5-lipoxygenase pathway, had no detectable effect on induction of CSF-1 RNA. PGE2 or dibutyryl cAMP treatment of HL-60 cells in the presence of TNF blocked the expression of CSF-1 mRNA in a dose-dependent manner. These findings suggest that the increase in CSF-1 RNA observed during TNF treatment is regulated, at least in part, by both transcriptional and posttranscriptional mechanisms, and that PGE2 and cAMP regulate transcriptional activation of the CSF-1 gene by TNF.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Rabinovitch M. Interferon induction in marrow-derived macrophages: regulation by L cell conditioned medium. J Cell Physiol. 1981 Sep;108(3):347–352. doi: 10.1002/jcp.1041080308. [DOI] [PubMed] [Google Scholar]
- Hansson A., Serhan C. N., Haeggström J., Ingelman-Sundberg M., Samuelsson B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1215–1222. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Horiguchi J., Sariban E., Kufe D. Transcriptional and posttranscriptional regulation of CSF-1 gene expression in human monocytes. Mol Cell Biol. 1988 Sep;8(9):3951–3954. doi: 10.1128/mcb.8.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Sherman M. L., Sampson-Johannes A., Weber B. L., Kufe D. W. CSF-1 and C-FMS gene expression in human carcinoma cell lines. Biochem Biophys Res Commun. 1988 Nov 30;157(1):395–401. doi: 10.1016/s0006-291x(88)80060-3. [DOI] [PubMed] [Google Scholar]
- Horiguchi J., Spriggs D., Imamura K., Stone R., Luebbers R., Kufe D. Role of arachidonic acid metabolism in transcriptional induction of tumor necrosis factor gene expression by phorbol ester. Mol Cell Biol. 1989 Jan;9(1):252–258. doi: 10.1128/mcb.9.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987 Apr;69(4):1259–1261. [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Ralph P., Kufe D. Expression of the macrophage specific colony-stimulating factor (CSF-1) during human monocytic differentiation. Biochem Biophys Res Commun. 1986 Dec 30;141(3):924–930. doi: 10.1016/s0006-291x(86)80131-0. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Sadowski S., Galavage M., Goldenberg M., Subers E., Kuehl F. A., Jr, Bonney R. J. Pharmacological effects of non-steroidal antiinflammatory agents on prostaglandin and leukotriene synthesis in mouse peritoneal macrophages. Biochem Pharmacol. 1983 Aug 1;32(15):2319–2322. doi: 10.1016/0006-2952(83)90179-x. [DOI] [PubMed] [Google Scholar]
- Imamura K., Sherman M. L., Spriggs D., Kufe D. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells. J Biol Chem. 1988 Jul 25;263(21):10247–10253. [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Tobler A. Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents. Mol Cell Biol. 1988 Aug;8(8):3432–3438. doi: 10.1128/mcb.8.8.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshihara Y., Neichi T., Murota S., Lao A., Fujimoto Y., Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984 Jan 17;792(1):92–97. [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Kurland J. I., Pelus L. M., Ralph P., Bockman R. S., Moore M. A. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci U S A. 1979 May;76(5):2326–2330. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. S., Gordon S. Secretion of plasminogen activator by bone marrow-derived mononuclear phagocytes and its enhancement by colony-stimulating factor. J Exp Med. 1979 Aug 1;150(2):231–245. doi: 10.1084/jem.150.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Synthesis by mouse peritoneal cells of G-CSF, the differentiation inducer for myeloid leukemia cells: stimulation by endotoxin, M-CSF and multi-CSF. Leuk Res. 1985;9(1):35–50. doi: 10.1016/0145-2126(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- Oster W., Lindemann A., Horn S., Mertelsmann R., Herrmann F. Tumor necrosis factor (TNF)-alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood. 1987 Nov;70(5):1700–1703. [PubMed] [Google Scholar]
- Peetre C., Gullberg U., Nilsson E., Olsson I. Effects of recombinant tumor necrosis factor on proliferation and differentiation of leukemic and normal hemopoietic cells in vitro. Relationship to cell surface receptor. J Clin Invest. 1986 Dec;78(6):1694–1700. doi: 10.1172/JCI112764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Warren M. K., Lee M. T., Csejtey J., Weaver J. F., Broxmeyer H. E., Williams D. E., Stanley E. R., Kawasaki E. S. Inducible production of human macrophage growth factor, CSF-1. Blood. 1986 Sep;68(3):633–639. [PubMed] [Google Scholar]
- Sakata A., Ida E., Tominaga M., Onoue K. Arachidonic acid acts as an intracellular activator of NADPH-oxidase in Fc gamma receptor-mediated superoxide generation in macrophages. J Immunol. 1987 Jun 15;138(12):4353–4359. [PubMed] [Google Scholar]
- Sariban E., Luebbers R., Kufe D. Transcriptional and posttranscriptional control of c-fos gene expression in human monocytes. Mol Cell Biol. 1988 Jan;8(1):340–346. doi: 10.1128/mcb.8.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Kasono K., Fujii Y., Kawakami M., Tsushima T., Shizume K. Tumor necrosis factor type alpha (cachectin) stimulates mouse osteoblast-like cells (MC3T3-E1) to produce macrophage-colony stimulating activity and prostaglandin E2. Biochem Biophys Res Commun. 1987 May 29;145(1):323–329. doi: 10.1016/0006-291x(87)91324-6. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Chen D. M., Lin H. S. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978 Jul 13;274(5667):168–170. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Tobler A., Johnston D., Koeffler H. P. Recombinant human tumor necrosis factor alpha regulates c-myc expression in HL-60 cells at the level of transcription. Blood. 1987 Jul;70(1):200–205. [PubMed] [Google Scholar]
- Trinchieri G., Kobayashi M., Rosen M., Loudon R., Murphy M., Perussia B. Tumor necrosis factor and lymphotoxin induce differentiation of human myeloid cell lines in synergy with immune interferon. J Exp Med. 1986 Oct 1;164(4):1206–1225. doi: 10.1084/jem.164.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Rosen M., Perussia B. Induction of differentiation of human myeloid cell lines by tumor necrosis factor in cooperation with 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1987 May 1;47(9):2236–2242. [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Release of arachidonate and reduction of oxygen. Independent metabolic bursts of the mouse peritoneal macrophage. J Biol Chem. 1986 Sep 5;261(25):11563–11570. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]