Abstract
Our recent studies have focused on cholesterol synthesis in mouse models for 7-dehydrosterolreductase (DHCR7) deficiency, also known as Smith-Lemli-Opitz syndrome. Investigations of such mutants have relied on tissue and blood levels of the cholesterol precursor 7-dehydrocholesterol (7DHC) and its 8-dehydro isomer. In this investigation by gas chromatography/mass spectrometry (GC/MS) we have identified and quantified cholesterol and its precursors (7DHC, desmosterol, lathosterol, lanosterol and cholest-7,24-dien-3β-ol) in mouse hair. The components were characterized and their concentrations were compared to those found in mouse skin and serum. Hair appeared unique in that desmosterol was a major sterol component, almost matching in concentration cholesterol itself. In DHCR7 deficient mice, dehydrodesmosterol (DHD) was the dominant hair Δ7 sterol.
Mutant mouse hair had much higher concentrations of 7-dehydrosterols relative to cholesterol than did serum or tissue at all ages studied. The 7DHC/C ratio in hair was typically about sevenfold the value in serum or skin and the DHD/D ratio was 100X that of the serum 7DHC/C ratio. Mutant mice compensate for their DHCR7 deficiency with maturity, and the tissue and blood 7DHC/C become close to normal. That hair retains high relative concentrations of the dehydro precursors suggests that the apparent up-regulation of Dhcr7 seen in liver is slower to develop at the site of hair cholesterol synthesis.
Keywords: Smith-Lemli-Opitz syndrome, SLOS, Hair Sterols, Sterol GC/MS, DHCR7 deficiency, Cholesterol biosynthesis
1. INTRODUCTION
Sterols and steroid levels in mice with mutations in 7-dehydrocholesterol reductase (DHCR7), the enzyme responsible for the human cholesterol deficiency syndrome Smith-Lemli-Opitz syndrome (SLOS) [1–4] have been studied by several groups. Predominantly, these studies have relied on serum and tissue for measurements of cholesterol and its precursors, primarily 7-dehydrocholesterol (7DHC). We have recently investigated the possibility of utilizing an alternative biological medium, hair, to develop in vivo, non-invasive assessments of enzyme activity.
Sterols have been previously found in human and animal hair [5–9] and are clearly the result of active cholesterol synthesis in the skin, hair follicle or adjacent sebaceous glands [10, 11]. Skin has long been known to be a major sterologenic tissue responsible for 25% of rat cholesterol synthesis [12]. Skin is also responsible for producing the 7DHC necessary for vitamin D synthesis [13, 14].
Cholesterol synthesis in the skin has been the focus of several recent studies. A mouse model of the human condition desmosterolosis, which is deficient in the enzyme that converts desmosterol to cholesterol (DHCR24), has been shown to have impaired epidermal development and a diminished number of hair follicles [15, 16]. Certain skin developmental disorders have also been demonstrated to be caused by buildup of apparently toxic precursors rather than deficiency of cholesterol itself [17].
In this paper we describe the analysis of hair and skin sterols and their levels in normal and Dhcr7 affected mice. The data reported suggest that the sterologenic synthetic pathway utilized for hair sterols is unique and independent of serum levels of cholesterol and it’s precursors.
2. MATERIALS AND METHODS
2.1. Materials. Origin of reference steroids used
The following reference compounds were obtained from Sigma Aldrich (sigmaaldrich.com): stigmasterol, cholesterol, 7-dehydrocholesterol, 5α-cholest-7-en-3β-ol (lathosterol), 5,24-cholestadien-3β-ol (desmosterol) and lanosterol.
2.2. Mice used in this study
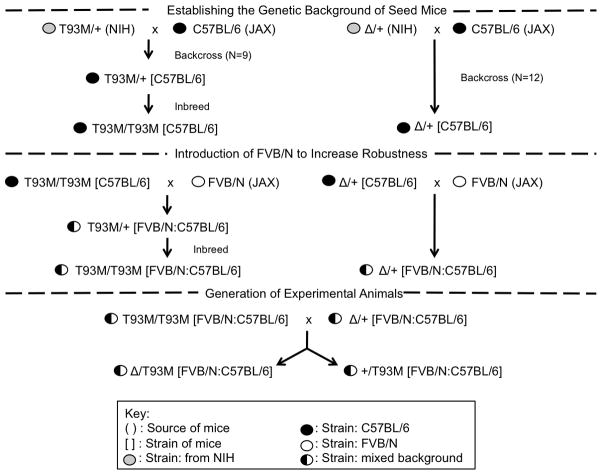
Animal work conformed to NIH guidelines and was approved by the Institutional Animal Care and Use Committee. All animals were maintained in an AALAC certified facility and were fed a normal, cholesterol-free chow (Teklad irradiated rodent diet 2918: Harlan, Madison, WI). Two strains of Dhcr7 affected mice were originally obtained from F.D.Porter (NIH). A null mutant contained a partial deletion of Dhcr7 (Δ)[18] and a hypomorphic mutant contained a point mutation (T93M) [19]. Mice with genotypes of T93M/T93M, Δ/T93M and Δ /Δ showed SLOS characteristics of increasing severity with Δ /Δ being lethal [18, 19]. As shown in Figure 2, the original mutants were backcrossed to C57BL/6 mice for several generations to provide a defined genetic background. In the C57BL/6 background, however, T93M/T93M and Δ/T93M had reduced viability making it very difficult to generate enough age-matched, affected mice for experiments. To increase mutant animal viability, the FVB/N background was introduced. FVB/N mice were originally developed for making transgenic mice, and this is a particularly robust and fertile inbred strain [20]. We reasoned (correctly as it turned out) that the introduction of FVB/N alleles would make mutant mice more resistant to the effects on viability caused by compromised cholesterol synthesis. Following the breeding scheme shown in Figure 2, we were able to generate viable SLOS animals (Δ/T93M) and phenotypically normal littermates (+/T93M). These mice, with a mixed C57BL/6 and FVB/N background, were used in all experiments. Genotypes of animals carrying Δ and T93M alleles were determined by PCR as described previously [18, 19].
Figure 2.
Breeding scheme for the generation of experimental animals. Original mutants with a mixed genetic background (strain 129 from embryonic stem cells and strain C57BL/6 from cross breeding) were made congenic in C57BL/6 by backcrossing. Due to an unexpected reduction of mutant viability in the C57BL/6 background, the FVB/N strain was introduced to provide viable mutants (Δ/T93M) and controls (+/T93M) with a mixed C57BL/6 and FVB/N background.
2.3. Collection of samples
Hair was clipped with scissors close to the skin. For blood collection, mice were anesthetized with 2.5% (v/v) Isoflurane using a VetEquip Funnel-Fill Vaporizer (www.vetequip.com) and 1 ml of blood was collected by cardiac puncture. The mice were sacrificed by cervical dislocation and skin was collected from the same area where hair had been clipped. Individual skin layers were not separated.
2.4. Sample preparation for sterol GC/MS
2.4.1. Hair
Hair (4–6 mg) was transferred to a glass tube; 4 ml methanol:chloroform (2:1) and 8 μg of stigmasterol were added and the sample was sonicated for 16 hours. The solvent was decanted and an additional 2 ml of extracting solvent was added. Samples were extracted again for 2 hours, and the solvent extracts were combined and dried.
2.4.2. Skin
Skin (20–30 mg) was extracted with 4 ml methanol:chloroform (2:1) and 48 μg of stigmasterol were added. The sample was extracted using the same protocol used for hair sterols, with the exception that only 1 ml of the total extract was analyzed.
2.4.3. Serum
Blood was cooled to 4 C to aid coagulation of red blood cells. Serum was separated by centrifugation for 3 minutes at 13000 rpm at ambient temperature. 0.8 μg of internal standard (stigmasterol) were added to 8 μl of serum for analysis.
2.4.4. Derivatization
The samples were then saponified by adding 500 μl of ethanol together with 300 μl of 33% KOH solution, followed by heating at 55 C for 45 minutes. After cooling to room temperature, 2 ml of water were added, and the samples were extracted twice with 2.5 ml of hexane by vortexing for 1 minute. The organic layers were transferred to fresh tubes, evaporated and derivatized with 50 μl of BSTFA (55° C for 45 minutes) to form the trimethylsilyl (TMS) derivatives. The derivatives were finally diluted with 500 μl of cyclohexane and transferred to autosampler vials for analysis by GC/MS. To minimize conversion of 7DHC to previtamin D3, all tubes were protected from light with foil covers. The whole procedure was conducted under minimum lighting conditions.
2.5. Analysis of sterols by GC/MS
The analysis was essentially as previously described for cholesterol and 7DHC [4] using an Agilent 5975 instrument (www.agilent.com). The sterols were separated on Agilent (chem.agilent.com) non-polar DB-1 or HP-5MS columns (30 m X 0.25 mm i.d., film thickness 0.25 μm). Both methylsilicone columns were similar, the latter additionally having 5% phenyl groups. The HP-5MS column seemed to have less bleed, but otherwise data obtained from each one was equivalent. The GC temperature was ramped as follows: initial 100ºC held for 1 minute, increased to 230 at 25ºC/min, increased to 290ºC at 2.5ºC/min and finally increased to 300ºC at 11ºC/min which was held for 2 minutes. The injector, transfer line, and ion source were kept at 260, 280 and 230ºC, respectively. The mass spectrometer was operated with electron impact ionization in SCAN mode. Retention indices were determined by co-injection of samples with a series of n-alkanes.
2.5.1. Preparation of calibration samples
7DHC and cholesterol were measured against an internal standard stigmasterol and standard curves prepared daily. Calibration mixtures were prepared by combining increasing amounts of 7DHC and cholesterol with fixed amount of stigmasterol, followed by derivatization. For hair, five concentration levels of the analytes (3–50ng injected) were present with 13 ng stigmasterol internal standard. For blood and skin with lower amounts of non-cholesterol sterols we used another 5 point calibration mixture; viz 13ng stigmasterol with 6–50 ng cholesterol and 0.5–10ng 7DHC (amount injected). The data plotted were the peak areas of fragment ion peaks from scanned data: 368 (for cholesterol), 325 (for 7DHC) and 394 (for stigmasterol). Other sterols were quantified by integrating peak areas on the TIC chromatograms and relating these to the peak area of the stigmasterol internal standard, for desmosterol the values were corrected using a measured response factor of 1.3, the remaining sterols were determined assuming a 1:1 response factor.
2.5.2 Validation of the method
The calibration curves were plotted by equal weighted least-squares linear regression analysis of the peak ratios of the analyte to the IS, against a nominal analyte concentration. The limit of detection (LOD) was determined as the lowest concentration measurable with a signal-to-noise ratio (S/N) >3 being 12 and 60 μg/g in hair tissue for cholesterol and 7DHC, respectively. The inter-day accuracy of the method was determined by measuring two standard solutions of different concentration in three separate analytical runs. Error was also calculated as a percentage; the nominal value was subtracted from the measured value and the difference was divided by the nominal value. Inter day variability for cholesterol and 7DHC was less than 6%. There will be systematic consistent errors in the quantitative values of DHD, lathosterol, lanosterol and cholest-7,24-dien-3β-ol which were quantified relative to stigmasterol from TIC peak integrations. The relative TIC response of individual sterols against stigmasterol remained constant although the precise relative response factor was not measured. Due to the similarity of structure of the monohydroxylated sterols, a working assumption of a response factor of unity is reasonable. For the steroids where response factors were determined they ranged from a high of 1.35 (cholesterol) to a low of 0.7 (DHC). The lack of absolute quantitative values should make little difference to the overall results produced from this study (and conclusions reached) where the same method is used consistently for all samples.
3. RESULTS
Three different experiments were carried out using Dhcr7 deficient mice (Δ/T93M, termed mutant or “affected”) and heterozygous +/T93M mice (termed normal or control), from the same litters. Experiment 1: Identification and quantitation of sterols in hair of 10 week old mice (Litter 1). Experiment 2: comparison of hair, skin and serum sterols in 7 week old animals (Litter 2). Experiment 3: comparison of sterol concentrations in hair of 3 week old (Litter 3) and 12 week old mice (Litter 4) with that of new-growth hair collected 2 weeks later.
3.1. Identification of sterols
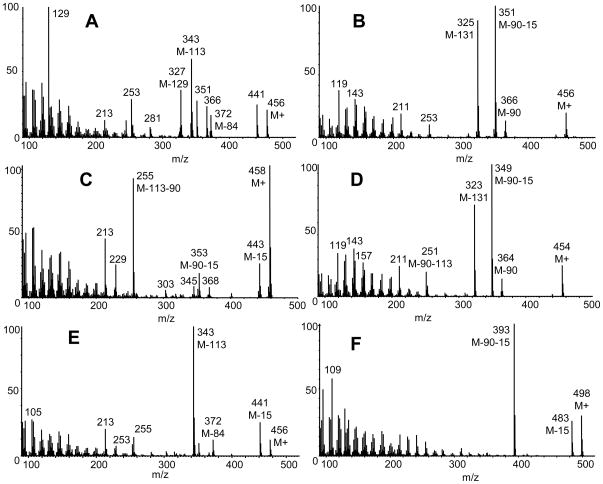
The following sections describe the criteria of GC/MS identification (mass spectrum and retention index) of mammalian sterols in hair from mutant and control animals. The mass spectra of isolated sterols as their trimethylsilylethers are shown in Figure 3. Two known phytosterols, campesterol and β-sitosterol were also identified but are not included here, neither was a dehydro methylcholesterol, a presumed phytosterol TMS ether of mass 470 Da.
Figure 3.
Mass spectra of major sterols found in hair as TMS derivatives. Panel (A) desmosterol; (B) 7DHC; (C) lathosterol; (D) 7DHD; (E) cholest-7,24-dien-3β-ol and (F) lanosterol.
3.1.1. Cholesterol (RI 3109)
This isolated sterol (as TMS derivative) had the same mass spectrum (well known, not illustrated) and retention index as reference cholesterol trimethylsilyl ether. The molecular ion is at m/z 458 and a prominent ion is formed by loss of trimethylsilanol m/z 368 (M-90). The base peak was seen at m/z 129, characteristic of 3β-hydroxy-5-ene steroids, and the corresponding fragment M-129 at m/z 329.
3.1.2. Desmosterol (RI 3137)
This isolated sterol had a mass spectrum (Fig. 3A) and retention index identical to available reference desmosterol trimethylsilyl ether. The base peak was seen at m/z 129, characteristic of 3β-hydroxy-5-ene steroids, and the corresponding fragment M-129 was present at m/z 327. Peaks at m/z 343 and 253 represent M-113, and M-113-90, respectively. These fragmentations are caused by the loss of the side chain together with two nuclear hydrogens [21]. Other prominent ions are at m/z 441 (M-15), 366 (M-90), 351 (M-15-90), and 372 (M-84).
3.1.3. 7-dehydrocholesterol (RI 3144)
This isolated sterol had the same mass spectrum (Fig. 3B) and retention index as reference 7DHC trimethylsilyl ether. The molecular ion is at m/z 456, and base peak at m/z 351 (M-90-15). The ion at m/z 325 represents loss of 131 Da characteristic of sterols with insaturation in 7 or 8 position. The 8-dehydrocholesterol isomer was also present in the mutant mice hair at low concentration. It has almost identical mass spectrum to 7DHC and co-elutes with cholesterol.
3.1.4. Lathosterol (RI 3155)
The compound illustrated in Figure 3C had the same mass spectrum and retention time index as the TMS derivative of reference lathosterol. The molecular ion and base peak is at m/z 458. The ion at m/z 255 probably represents loss of trimethylsilanol and side chain (M-90-113). The origin of the ions at m/z 303 (M-155) and m/z 213 (M-155-90) were not determined.
3.1.5. 7-Dehydrodesmosterol (7DHD) (RI 3171)
The mass spectrum illustrated in Figure 3D would be appropriate for the TMS derivative of 7-dehydrodesmosterol. A reference steroid was not available. The spectrum is almost identical of that of 7DHC with major ions 2 Da less in mass. The molecular ion is at m/z 454 with base peak at m/z 349 (M-90-15) and a prominent ion at m/z 323 (M-131). These three ions have approximately the same abundance as the equivalent (but 2 daltons heavier) ions in 7DHC. Thus it can be assumed that the isolated steroid has the same ring structure as 7DHC, the mass difference being due to the presence of the double bond at C-24. Although from the mass spectrum 8DHD would also be a possibility since it essentially would share the spectrum of 7DHD this sterol would have a lower retention index, based on the 7 and 8DHC comparison. In addition the peak shape of the isolated sterol was broad with a frontal slope, a distinctive feature of 7-dehydro but not 8-dehydro sterols and steroids [22]. Small amounts of similar sterols have been tentatively identified in serum and feces from SLOS patients [23, 24]. Wolf et al [25] show spectra of sterol trienols (M+ 454) in serum of rats treated with 7-dehydrocholesterol inhibitors. They suggest the spectra are consistent with 7-and 8-dehydrodesmosterol, but in contrast to our study, the spectra do not reproduce the characteristic ion pattern seen in 7- and 8-dehydrocholesterol.
3.1.6. Cholest-7,24-dien-3β-ol (RI 3188)
This steroid (Fig. 3E) was identified as cholest-7,24-dien-3β-ol TMS ether by comparison to the spectrum published by Gerst et al [22] and the relative retention time published by Ruan and co-workers [26]. The molecular ion is at m/z 456. The base peak is at m/z 343 (M-113). Other significant ions were at m/z 441 (M-15), m/z 372 (M-84, also present in desmosterol), m/z 213 (M-243), m/z 253 and m/z 255.
3.1.7. Lanosterol (RI 3277)
This isolated sterol (Fig. 3F) had the same mass spectrum and retention index as reference lanosterol TMS ether. The molecular ion is at m/z 498 and the base peak is at m/z 393 (M-90-15). There is a prominent ion at m/z 109.
3.2. Quantitation in Mouse Samples
3.2.1. Experiment 1
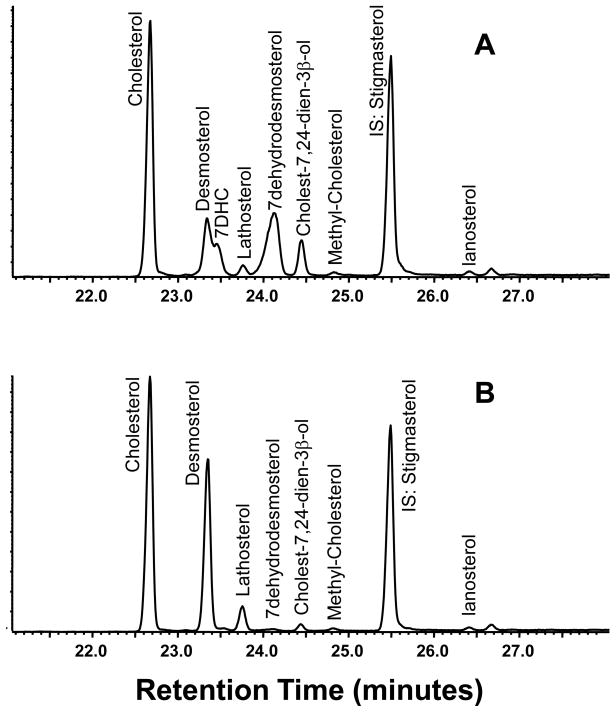
In a pilot experiment we analyzed sterols from the hair of 4 affected mice aged 10 weeks and 4 of their heterozygote (control) littermates. From the chromatogram (Fig. 4A and B) control animals have a profile dominated by cholesterol and desmosterol with a lesser amount of lathosterol and other sterols. By contrast, hair from affected animals has a high relative concentration of sterols with Δ7 unsaturation including 7DHC, 7DHD and 7,24-cholestadien-3β-ol (Fig. 4A). Included in Table 1 are the concentrations, the amount of each relative to the total of quantified mammalian sterols, and the DHC/C and DHD/D ratios. These ratios were highly elevated in affected mice compared to their control littermates, particularly so for 7DHD/DHD. Interestingly, the values for this ratio were sevenfold higher than those of the DHC/C ratios.
Figure 4.
Segments of total-ion-current (TIC) chromatograms of sterols in hair (as TMS derivatives). Panel 4A is of a 10 week old affected animal and 4B corresponds to a control animal from same litter.
TABLE 1.
Sterols in hair of Litter 1 mice at 10 weeks of age.
| Sterol and Ratios | Concentration (μg/g) | Percentage of total sterols | ||
|---|---|---|---|---|
| SLOS1 | Normal1 | SLOS2 | Normal2 | |
| Cholesterol | 1500±100 | 2100±200 | 39±2 | 54.3±0.3 |
| 7DHC | 600±30 | <1 | 15±2 | <0.02 |
| Ratio 7DHC/C | 0.40±0.05 | NA | NA | NA |
| Desmosterol | 350±603 | 1500±100 | 9±1 | 38±2 |
| 7DHD | 940±703 | 11±5 | 25±2 | 0.3±0.1 |
| Ratio 7DHD/D | 2.8±0.5 | 0.007±0.004 | NA | NA |
| Lathosterol | 220±503 | 230±60 | 6±1 | 6±1 |
| Cholest-7,24-dien-3β-ol | 170±403 | 46±20 | 4±1 | 1.2±0.5 |
| Lanosterol | 15±153 | 15±15 | 0.4±0.4 | 0.4±0.4 |
The numbers given are for the concentration (mean±SD) and ratios for four control and four affected mice.
Individual sterols as percentage of total measured sterols present for the same mice (mean±SD).
Absolute values for 7DHD, lathosterol, cholest-7,24-dien-3β-ol and lanosterol are estimates as TIC peak areas were measured against stigmasterol using a response factor of unity, except for desmosterol where calculated response factor was 1.3.
Attachment of sterols to hair
Are hair sterols loosely coated on hair or are they strongly bonded or internalized? An experiment was under taken to determine if sterols could be removed by washing. For four mice (two affected and two control), two samples of hair were analyzed. One sample was extracted as previously described, but the second was washed prior to extraction. This was carried out with 5 ml of a solution of SDS (sodium dodecyl sulphate, 0.5%) for 10 minutes, vortexing the sample every 3 minutes. Hair was rinsed three times with water and extracted as previously described. The sterol concentration in hair proved to be essentially unchanged by prior washing which suggests that sterols may be internalized within the shaft and/or strongly surface bonded.
3.2.2. Experiment 2: comparison of hair, skin, and serum sterols
At 7 weeks of age 3 control and 3 affected mice from Litter 2 were sacrificed. Hair was collected from two different areas of the back, and skin was excised from the same areas. Blood was collected and serum separated. The results of sterol analysis of these samples are given in Table 2 and show that the profile of sterols in the hair samples from the contol and affected animals was essentially the same as in the previous experiment, desmosterol and dehydrodesmosterol being major constituents in control and affected mice, respectively. In serum, cholesterol was by far the dominant sterol and 7DHC was not greatly elevated even in mutant animals. The DHC/C ratio was 0.02–0.03 compared to values in hair of 0.2–0.3. Important hair sterols such as 7DHD, cholest-7,24-dien-3β-ol, lathosterol and lanosterol were below our detection limit for serum.
TABLE 2.
Sterols in hair, skin and serum of Litter 2 mice at 7 weeks of age.
| Sterol and Ratio | Hair (μg/g) | Skin (μg/g) | Serum (μg/ml) | |||
|---|---|---|---|---|---|---|
| SLOS1 | Normal1 | SLOS | Normal | SLOS | Normal | |
| Cholesterol | 1500±100 | 2200±100 | 840±30 | 790±80 | 430±70 | 510±30 |
| 7DHC | 730±50 | <1 | 70±30 | <1 | 16±10 | <0.1 |
| Ratio 7DHC/C | 0.48±0.07 | NA | 0.08±0.04 | NA | 0.04±0.04 | NA |
| Desmosterol | 550±902 | 2400±160 | <1 | <1 | <0.1 | <0.1 |
| 7DHD | 1300±1502 | 50±10 | <1 | <1 | <0.1 | <0.1 |
| Ratio 7DHD/D | 2.4±0.2 | 0.020±0.03 | NA2 | NA | NA | NA |
| Lathosterol | 160±802 | 200±30 | 110±90 | 270±150 | <0.1 | <0.1 |
| Cholest-7,24-dien-3β-ol | 300±302 | 110±30 | <1 | <1 | <0.1 | <0.1 |
| Lanosterol | 100±802 | 80±60 | <1 | <1 | <0.1 | <0.1 |
The numbers given are for the concentration (mean±SD) and ratios for three control and three affected mice.
Absolute values for 7DHD, lathosterol, cholest-7,24-dien-3β-ol and lanosterol are estimates as TIC peak areas were measured against stigmasterol using a response factor of unity, except for desmosterol where calculated response factor was 1.3.
NA: Not applicable.
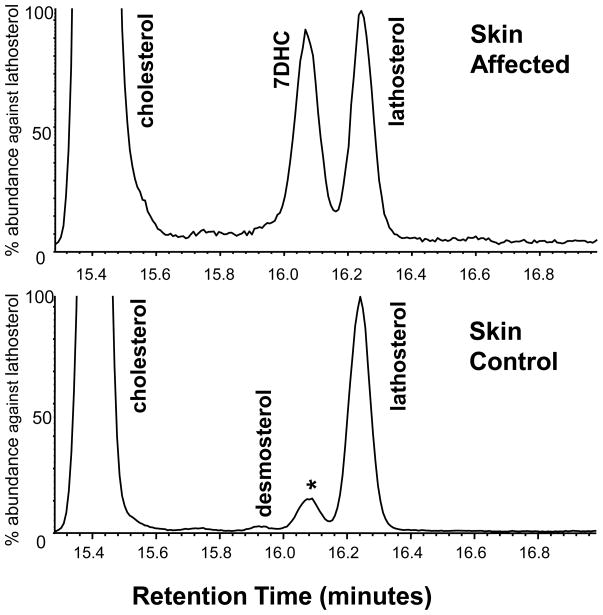
In skin, cholesterol was also dominant but lathosterol was present in all samples at low concentration. Desmosterol was only measurable in a single animal (of 12 studied), and then only in a trace amount, representing about 0.6% of the amount of cholesterol. Enlarged sections of skin chromatograms shown in Figure 5 illustrate the trace amount of desmosterol present as well as the absence of cholest-7,24-en-3β-ol and DHD which are prominent components of hair.
Figure 5.
Segments of TIC chromatograms of skin sterols targeted to show minor sterols. The small desmosterol peak was only found in this one control sample and no peaks were seen for 7DHD or 5,24-cholestadien-3β-ol. In the control animal, the peak marked by an asterisk with the retention time of 7DHC was an unidentified dehydro-methylcholesterol, presumably of plant origin.
3.2.3. Experiment 3: sterols in second growth hair
Sterols bound to, or incorporated in hair probably have a degree of permanence; once present they are not easily removed, as illustrated from the washing experiment. Thus, the sterol concentration in hair from the 7 and 10 week old animals (either affected or control) described above is a composite of sterols laid-down in the first days of hair growth (at 1–2 weeks of age in mice) and each week thereafter. Hair harvested at a single time point would not accurately reflect the amount of individual sterols just synthesized. For this reason we determined the sterols synthesized in a given period of time by analyzing new growth hair from a previously shaved area.
In this experiment two sets of mouse litters were studied of age 3 (Litter 3) and 12 weeks (Litter 4), respectively. At the outset, hair for sterol analysis was cut from one area of the animals back (baseline values); two weeks later re-grown hair was harvested from the same area, as well as original growth hair from an adjacent area. Thus, the following samples were obtained and analyzed: Litter 3: 3-week-old hair, 5-week-old hair, and hair grown between the 3rd and 5th weeks (new growth); and Litter 4, 12-week-old hair, 14-week-old hair and hair grown between the 12th and 14th weeks. Blood was also drawn and serum sterols quantified.
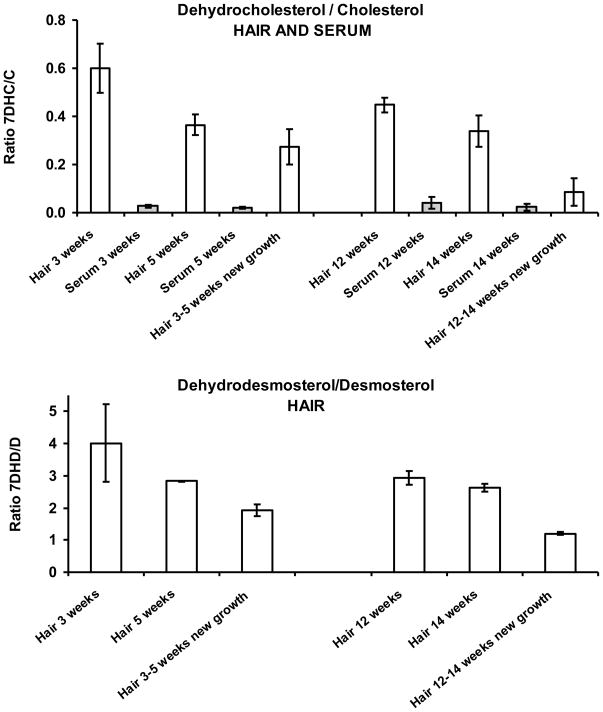
The results are shown in Figure 6. The upper panel shows the DHC/C ratios and the lower panel shows the corresponding values for the DHD/D ratios, solely obtained for hair because desmosterol and dehydrodesmosterol were below our detection limits in serum. The values for DHC/C and DHD/D in new-growth hair are lower (by about 50%) than that of the first-growth hair showing that there is a decreasing ratio of hair DHC/C and DHD/D with age, possibly a result of Dhcr7 upregulation.
Figure 6.
Ratios of sterol concentrations. Upper panel: hair and serum DHC/C ratios of affected animals in two separate litters, initially studied at 3 and 12 weeks, respectively, then two to four weeks later. Lower panel: hair DHD/D ratio.
We noted during this experiment that second growth hair grew much slower in affected animals than in littermate controls.
4. DISCUSSION
This study describes the major sterols present in hair of phenotypically normal mice (heterozygote for the T93M mutation) and DHCR7 deficient mice, and compares them, qualitatively and quantitatively to those present in skin and serum. The results are summarized in Table 1. The profile of major sterols in hair is more complex than in blood or tissue. We and other investigators have found that while there are quantitatively low amounts of cholesterol precursors (<1% of cholesterol) in the livers and brains of normal mice [17, 27], hair is a rich source of such compounds. Of particular interest, in our control mice high levels of desmosterol were detected and the concentrations were often on par with those of cholesterol. This would suggest that at the biosynthetic site responsible for hair cholesterol synthesis, CYP51 is a more successful competitor for lanosterol than 24-reductase (DHCR24). Other 24-dehydro sterols were also found, including cholest-7,24-dien-3β-ol and lanosterol.
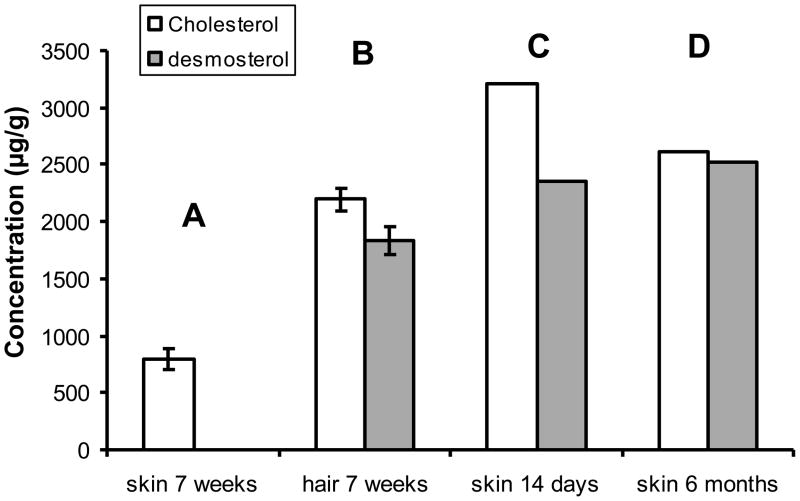
In the skin of normal animals we found that the relative concentrations of sterols were similar to those in serum and other tissues. As expected, the cholesterol concentration was orders of magnitude greater than that of precursor sterols. We found the cholesterol/desmosterol ratio was more than 1000:1. Similarly, little desmosterol was also reported by Mirza et al [15] who found the cholesterol/desmosterol ratio in skin (epidermis) to be greater than 100:1. By contrast, Evers and co-workers [17], measured skin sterols in normal mice and reported very high desmosterol concentrations. Their cholesterol/desmosterol ratio at about 1.2:1 was similar to the values we found in hair (Fig. 7). Currently we have no explanation for this discrepancy between studies as the methodology used for sterol characterization and quantitation was comparable between our laboratories.
Figure 7.
Desmosterol in hair and skin. Histogram of cholesterol and desmosterol levels in skin: A) skin concentrations in 7 week old mice (our studies), desmosterol concentration below limit of detection; B) hair concentrations of cholesterol and desmosterol in 7 week old mice (our studies). Panels C and D shows results reported by Evers et al [17]; panel C are skin concentrations of cholesterol and desmosterol in control mice at two weeks of age, and panel D skin concentrations at age six months in animals that had had topical statin treatment prior to 2 weeks of age.
Overall, skin is a highly sterologenic organ responsible for a quarter of total cholesterol synthesis in rats [28]. Both dermal and epidermal layers have high concentrations of sterols, and have been implicated in their synthesis [28–30]. Exactly where hair sterols are synthesized has yet to be determined but we suspect it to be in the hair follicle or in the adjacent sebaceous gland. The process of washing the hair with SDS prior to solvent extraction removed very little sterol indicating that the sterols are either embedded or strongly attached. This would suggest that the sterols are incorporated into the hair close to its origin rather than just coating the hair after it has grown. Sterologenic enzymes such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA) and DHCR24 are strongly expressed in hair follicles [10, 15, 31]. In contrast to humans, mice have high cholesterol content in their sebaceous glands so given the close proximity of these glands to the newly-formed hair shaft they also could be the source of hair sterol.
The high levels of desmosterol found in hair of control mice would lead one to expect high levels of 7-dehydrodesmosterol (7DHD) in DHCR7 deficient mice, and this is indeed the case (Fig. 4B and Tables 1 and 2). This is our first identification of this sterol as it was not found in previous studies of the brain or liver of DHCR7 deficient mice (although it is likely to be present in these tissues in low concentrations). In DHCR7 deficient mice, the hair DHD/D ratio is remarkably high relative to the DHC/C ratio, which is interesting as it suggests the DHCR7 enzyme has less activity towards 24-dehydrosterols.
The DHCR7 deficient mouse model used for these experiments retains some 7-dehydrocholesterol reductase activity, which still allows a reduced rate of cholesterol synthesis. In newborn affected mice the liver DHC/C ratio is about 0.3, but this drops rapidly during suckling. There is a sharp increase after weaning, but then the ratio gradually diminishes with ageing. Cholesterol levels at one year of life are normal and DHC levels are low [4]. The brain DHC/C ratio is higher in newborns (about 1.0), but also decreases with age and eventually approaches near normal values (0.01) [4].
The sterol synthesis of hair is very different from that of liver, the major source of serum cholesterol. It was mentioned above that high concentrations of desmosterol in hair indicate limited desmosterol reductase (DHCR24) activity at the site of hair sterol synthesis, but DHCR7 activity within this site is also much reduced compared to liver. This gives rise to high hair DHC/C and DHD/D ratios which persist for many weeks, and even remain high when determined for new-growth hair at 12–14 weeks of age. It may be of clinical interest to measure sterols in hair from SLOS patients to see if this medium contains 7 and 8DHC (and DHD) and whether these may be useful for diagnosing the disorder, particularly in mild cases which have borderline normal serum DHC/C ratios.
Recently there has been a renewed interest in cholesterol biosynthesis in skin and its regulation, particularly in regards to the formation and influence of its precursors. Mirza et al [15, 16] have studied the Dhcr24 knockout mouse, created to study the human disorder desmosterolosis [32]. They found that newborn pups with this enzyme deficiency had disordered epidermal and skin follicle development and that the animals died soon after birth. The epidermis of these animals had high concentrations of cholesterol precursors but essentially no cholesterol. Evers and co-workers [17] investigated knockout mice for two regulatory proteins, Insig-1 and Insig-2, which are regulatory proteins for cholesterol biosynthesis. These were tissue specific knockouts, the epidermis being one of the mutant tissues. The animals exhibited a marked defect in hair growth and other abnormalities and high levels of cholesterol precursors were found in skin. The skin and hair abnormalities were completely reversed by topical statin treatment. Skin cholesterol was plentiful in these animals so its deficiency could not be blamed for the abnormalities. Evers and colleagues [17] concluded that one or more components of the precursor sterols present in excess had toxicity to skin development. The mechanism by which this occurs remains unknown, but interference of these compounds with the sonic hedgehog (shh) signaling system certainly is a candidate [17, 33]. It is known than sonic hedgehog is involved in skin development [34, 35]. These findings clearly have implications to SLOS and the other cholesterol-biosynthetic-defect malformation syndromes. There is a continuing debate as to whether its cholesterol deficiency or precursor toxicity that is causative of the developmental phenotype.
Figure 1.
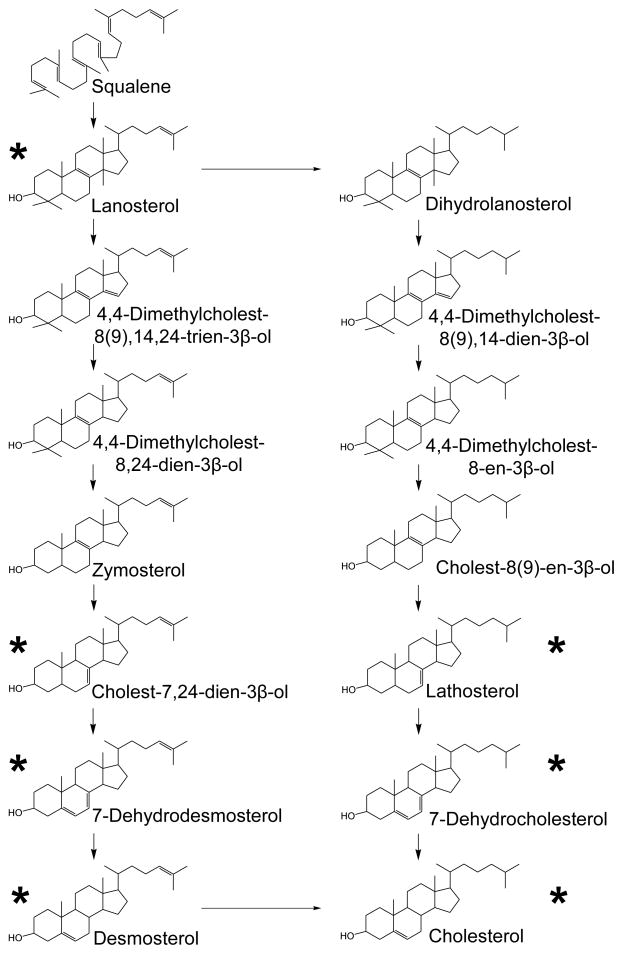
The post–squalene synthesis of cholesterol. Two synthesis pathways are shown, the Kandutsch-Russel pathway at left and Bloch pathway at right. The sterols marked with an asterisk were identified and measured in this study.
Acknowledgments
This work was supported by National Institutes of Health Grant R01HD053036 (CS) and a grant from the Smith-Lemli-Opitz/RSH Foundation (GW). CS is also supported by MRC Expermental Grant – DLAC.RRAK11881 and Welcome Trust Programme Grant – DLAC.RCHX13057. Dr. Serra was awarded a Beatriu de Pinós postdoctoral fellowship from Generalitat de Catalunya (2007 BP-A 00101). Amb el suport del Comissionat per a Universitats i Recerca del Departament d’Innovació, Universitats i Empresa de la Generalitat de Catalunya. We thank Kevin Hua for technical help. We are grateful to Drs F. D. Porter and Christopher Wassif of the NIH for providing the seed mice used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzky BU, Witsch-Baumgartner M, Erdel M, et al. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci U S A. 1998;95(14):8181–6. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassif CA, Maslen C, Kachilele-Linjewile S, et al. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1998;63(1):55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterham HR, Wijburg FA, Hennekam RC, et al. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63(2):329–38. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcos J, Shackleton CH, Buddhikot MM, Porter FD, Watson GL. Cholesterol biosynthesis from birth to adulthood in a mouse model for 7-dehydrosterol reductase deficiency (Smith-Lemli-Opitz syndrome) Steroids. 2007;72(11–12):802–8. doi: 10.1016/j.steroids.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wertz PW, Downing DT. Integral lipids of mammalian hair. Comp Biochem Physiol B. 1989;92(4):759–61. doi: 10.1016/0305-0491(89)90264-2. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides N, Rothman S. Studies on the chemical composition of human hair fat. I. The squalene-cholesterol relationship in children and adults. J Invest Dermatol. 1952;19(6):389–91. doi: 10.1038/jid.1952.113. [DOI] [PubMed] [Google Scholar]
- 7.Masukawa Y, Narita H, Imokawa G. Characterization of the lipid composition at the proximal root regions of human hair. J Cosmet Sci. 2005;56(1):1–16. [PubMed] [Google Scholar]
- 8.Masukawa Y, Tsujimura H, Imokawa G. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;823(2):131–42. doi: 10.1016/j.jchromb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Wertz PW, Downing DT. Integral lipids of human hair. Lipids. 1988;23(9):878–81. doi: 10.1007/BF02536208. [DOI] [PubMed] [Google Scholar]
- 10.Smythe CD, Greenall M, Kealey T. The activity of HMG-CoA reductase and acetyl-CoA carboxylase in human apocrine sweat glands, sebaceous glands, and hair follicles is regulated by phosphorylation and by exogenous cholesterol. J Invest Dermatol. 1998;111(1):139–48. doi: 10.1046/j.1523-1747.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Laubner D, Breitling R, Adamski J. Embryonic expression of cholesterogenic genes is restricted to distinct domains and colocalizes with apoptotic regions in mice. Brain Res Mol Brain Res. 2003;115(1):87–92. doi: 10.1016/s0169-328x(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 12.Feingold KR, Brown BE, Lear SR, Moser AH, Elias PM. Localization of de novo sterologenesis in mammalian skin. J Invest Dermatol. 1983;81(4):365–9. doi: 10.1111/1523-1747.ep12519974. [DOI] [PubMed] [Google Scholar]
- 13.Glossmann HH. Origin of 7-Dehydrocholesterol (Provitamin D) in the Skin. 2010;130(8):2139–2141. doi: 10.1038/jid.2010.118. [DOI] [PubMed] [Google Scholar]
- 14.Rossi M, Federico G, Corso G, et al. Vitamin D status in patients affected by Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2005;28(1):69–80. doi: 10.1007/s10545-005-3676-8. [DOI] [PubMed] [Google Scholar]
- 15.Mirza R, Hayasaka S, Takagishi Y, et al. DHCR24 gene knockout mice demonstrate lethal dermopathy with differentiation and maturation defects in the epidermis. J Invest Dermatol. 2006;126(3):638–47. doi: 10.1038/sj.jid.5700111. [DOI] [PubMed] [Google Scholar]
- 16.Mirza R, Qiao S, Murata Y, Seo H. Requirement of DHCR24 for postnatal development of epidermis and hair follicles in mice. Am J Dermatopathol. 2009;31(5):446–52. doi: 10.1097/DAD.0b013e318196f10c. [DOI] [PubMed] [Google Scholar]
- 17.Evers BM, Farooqi MS, Shelton JM, et al. Hair growth defects in Insig-deficient mice caused by cholesterol precursor accumulation and reversed by simvastatin. J Invest Dermatol. 2010;130(5):1237–48. doi: 10.1038/jid.2009.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassif CA, Zhu P, Kratz L, et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith--Lemli--Opitz syndrome. Hum Mol Genet. 2001;10(6):555–64. doi: 10.1093/hmg/10.6.555. [DOI] [PubMed] [Google Scholar]
- 19.Correa-Cerro LS, Wassif CA, Kratz L, et al. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum Mol Genet. 2006;15(6):839–51. doi: 10.1093/hmg/ddl003. [DOI] [PubMed] [Google Scholar]
- 20.Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88(6):2065–9. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks CJ, Horning EC, Young JS. Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids. 1968;3(5):391–402. doi: 10.1007/BF02531277. [DOI] [PubMed] [Google Scholar]
- 22.Gerst N, Ruan B, Pang J, Wilson WK, Schroepfer GJ., Jr An updated look at the analysis of unsaturated C27 sterols by gas chromatography and mass spectrometry. J Lipid Res. 1997;38(8):1685–701. [PubMed] [Google Scholar]
- 23.Kelley RI. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta. 1995;236(1):45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- 24.Batta AK, Tint GS, Salen G, Shefer S, Irons M, Elias ER. Identification of 7-dehydrocholesterol and related sterols in patients with Smith-Lemli-Opitz syndrome. Am J Med Genet. 1994;50(4):334. [Google Scholar]
- 25.Wolf C, Chevy F, Pham J, et al. Changes in serum sterols of rats treated with 7-dehydrocholesterol-delta 7-reductase inhibitors: comparison to levels in humans with Smith-Lemli-Opitz syndrome. J Lipid Res. 1996;37(6):1325–33. [PubMed] [Google Scholar]
- 26.Ruan B, Wilson WK, Pang J, et al. Sterols in blood of normal and Smith-Lemli-Opitz subjects. J Lipid Res. 2001;42(5):799–812. [PubMed] [Google Scholar]
- 27.Fon Tacer K, Pompon D, Rozman D. Adaptation of cholesterol synthesis to fasting and TNF-alpha: Profiling cholesterol intermediates in the liver, brain, and testis. J Steroid Biochem Mol Biol. 2010;121(3-5):619–625. doi: 10.1016/j.jsbmb.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Feingold KR, Wiley MH, MacRae G, et al. De novo sterologenesis in the intact rat. Metabolism. 1983;32(1):75–81. doi: 10.1016/0026-0495(83)90160-9. [DOI] [PubMed] [Google Scholar]
- 29.Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–22. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappas A. Epidermal surface lipids. Dermatoendocrinol. 2009;1(2):72–6. doi: 10.4161/derm.1.2.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brannan PG, Goldstein JL, Brown MS. 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human hair roots. J Lipid Res. 1975;16(1):7–11. [PubMed] [Google Scholar]
- 32.Andersson HC, Kratz L, Kelley R. Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am J Med Genet. 2002;113(4):315–9. doi: 10.1002/ajmg.b.10873. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104(7):855–64. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006;15(9):667–77. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 35.Glotzer DJ, Zelzer E, Olsen BR. Impaired skin and hair follicle development in Runx2 deficient mice. Dev Biol. 2008;315(2):459–73. doi: 10.1016/j.ydbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]