Abstract
There are eight naturally occurring forms of the dietary antioxidant vitamin E. Of these, only α-tocopherol is retained at high levels in vertebrate plasma and tissues. This selectivity is achieved in part by the action of the hepatic alpha tocopherol transfer protein (TTP), which facilitates the selective incorporation of dietary α-tocopherol into circulating lipoproteins. We examined the effects of vitamin E on TTP expression in cultured hepatocytes. Treatment with vitamin E brought about a time- and dose-dependent increase in the steady-state levels of TTP. This stabilization was caused by α-tocopherol-induced attenuation of the ubiquitination of TTP and its subsequent degradation by the proteasome. In vitro, vitamin E protected TTP from proteolytic degradation by trypsin, suggesting ligand-induced changes in protein conformation. Cell fractionation studies showed that TTP is distributed between the cytosolic and the membranous organelle fraction, and that tocopherol induced the translocation of some TTP from the cytosol to the organelle fraction. Furthermore, vitamin E markedly attenuated the degradation of organelle-bound TTP. These findings suggest that vitamin E imparts a distinct conformation on TTP that is associated with localization to a specific cellular compartment, where the protein is less susceptible to proteasomal degradation.
Keywords: α-tocopherol, vitamin E, tocopherol transfer protein, antioxidant, proteasome
Alpha tocopherol is a lipophilic, plant-derived neutral lipid that functions as chain breaking antioxidant in biological membranes (1). The effective radical-trapping ability of vitamin E is thought to underlie the strict biological requirement for this nutrient and its protective actions against a number of human pathologies. While plants synthesize eight forms of vitamin E (α-, β-, γ- and δ- tocopherols and tocotrienols), animals retain primarily α-tocopherol as their lipid-phase antioxidant (2).
Tissue distribution of α-tocopherol shares many steps with the transport of other neutral lipids. After ingestion, the vitamin is taken up by enterocytes and packaged in chylomicra that deliver the vitamin to the liver. Delivery from the liver to peripheral tissues is mediated by circulating low- and high-density lipoprotein particles (3, 4). The only known transport mechanism that is specific to α-tocopherol occurs in the liver, where the alpha tocopherol transfer protein (TTP) selectively binds this vitamin and facilitates its secretion from hepatocytes (4, 5). Heritable mutations that perturb TTP function in humans are the only known cause for ataxia with vitamin E deficiency (AVED, OMIM 600415), a familial disorder characterized by vitamin E deficiency and progressive loss of movement coordination (6,7). Mice in which expression of the ttpA gene is disrupted are vitamin E deficient, and display neurological symptoms similar to those observed in AVED patients (8,9). Progression of the AVED pathology can be sometimes prevented by high-dose vitamin E supplementation in both Ttp−/− mice and in human patients, further underscoring the relationship between TTP, α-tocopherol levels, and CNS health. To-date, TTP is the only known protein that specifically regulates body-wide levels of α-tocopherol.
The molecular mechanisms by which TTP influences trafficking of vitamin E in the liver has received significant attention (10–14). The protein is expressed in parencymal cells of the liver, and is believed to bind newly arrived α-tocopherol in the endocytic compartment. Through a poorly-understood process, TTP then facilitates the transfer of α-tocopherol to transport vesicles that deliver the vitamin to its site of secretion in the hepatocyte plasma membrane (12, 13). Despite the central roles that TTP plays in maintaining vitamin E adequacy, very little is known regarding the mechanisms that regulate the expression and biochemical activity of the protein. We report here our findings on the factors that govern the stability of TTP in cultured cells, and on its regulation by vitamin E.
Materials and Methods
Reagents
RRR-α-tocopherol was purchased from Cole-Palmer. Cycloheximide and chloroquine were purchased from Sigma Aldrich. Doxycycline and MG132 were obtained from Calbiochem. Clasto-lactacystin β-lactone, ubiquitine aldehyde and N-ethylmalemide (NEM) were purchased from Boston Biochemicals. Except where noted, TTP was visualized on Western blots using monoclonal antibodies directed against the protein’s amino-terminal hemagglutinin tag (HA.11, Covance Inc.; see 12). Anti-ubiquitin and anti-actin antibodies were purchased from Biomol Inc. and Cell Signaling Inc., respectively. The 12D7 anti-TTP antibody was a generous gift of Hiroyuki Arai (University of Tokyo, Tokyo, Japan). Immortalized human hepatocytes (15) were the generous gift of Ranjit Ray (Saint Louis University).
Cell Culture
HepG2-TetOn-TTP cells stably expressing HA-tagged TTP under the control of the inducible TetOn promoter were described earlier (12). Cells were maintained in Dulbeco Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Atlanta Biologicals) in the presence of G418 (Gibco) and hygromycin (Invitrogen) at 100 µg/ml each. TTP expression was induced by adding 1 µg/ml doxycycline to the growth media 48 hrs prior to cell lysis. Alpha-tocopherol was added to the culture media (100 µM final concentration, unless specified otherwise) from ethanolic stocks 48 hrs prior to cell lysis. Equal volumes of ethanol were added to control cultures. Proteolysis inhbitors were added from DMSO stocks to 100 µM (chloroquine), or 10 µM (MG132 and Clasto-lactacystin β-lactone) for the indicated durations. Immortalized human hepatocytes (IHH) were cultured in DMEM supplemented with 10% defined fetal bovine serum containing < 0.1 µM α-tocopherol (HyClone). All other methods were described earlier (12, 16). Unless otherwise indicated, cell lysates were prepared by incubation for 10 min on ice in 20 mM HEPES (7.4), 1mM EDTA, 150 mM NaCl, 1% Nonident-P40 (NP-40), 20 mM NaF, 20 mM β-glycerolphosphate, 1 mM sodium vanadate, and 10 µg/ml of each leupeptin and aprotonin, followed by microcentrifgugation at 14,000 × g.
Ubiquitination assays
Cells were treated with the proteasome inhibitor MG132 for 5–6 hrs prior to termination of the experiment. Cells were lysed in 20 mM HEPES (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 20 mM NaF, 20 mM β-glycerolphosphate 1 mM EDTA, 1.0 mM phenylmethylsulfonyl fluoride, and 10 µg/ml each of leupeptin and aprotinin, 1 mM sodium vanadate, 1 µM ubiqitin aldehyde and 1 mM N-ethyl-maleamide. TTP was immunoprecipitated using sepharose beads conjugated to anti-HA antibodies (Covance Inc.). Precipitates were resolved on SDS-PAGE, and ubiquitination of TTP was detected by western blotting using an anti-ubiquitin antibodies after autoclaving the membrane for 30 minutes.
In vitro proteolysis
Recombinant TTP was purified from over-expressing E. coli as previously described (16). TTP (12.5 µM protein in 20 mM Tris pH 8.0, 150 mM NaCl) was incubated with 100 µM RRR-α-tocopherol (or ethanol control) in silicon-coated tubes for three hours on ice. Trypsin (7 µM) was added to the reaction mixture and incubated for the indicated duration at 37°C. The reaction was terminated by boiling in Laemmeli buffer, after which samples were resolved on SDS-PAGE and visualized by Coomassie staining. Signal intensity was measured by densitometry using Scion image (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Cell fractionation
Cells were trypsinized and washed three times by centrifugation at 6,800 × g in cold PBS. Pellets were resuspended in 50mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.2 mM phenylmethylsulfonyl fluoride and 10 µg/ml each of leupeptin and aprotinin, and lysed by 5 cycles of freeze-thawing. Centrifugation at 24,000 × g for 1 hr at 4°C was used to separate the cytosol (supernatant) and the organelle (pellet) fractions.
RESULTS
While characterizing the expression of ectopically expressed TTP in cultured HepG2 cells, we observed that the steady-state expression levels of the protein increased by 2–3-fold when vitamin E was included in the culture media (Figure 1A). The increase in TTP expression was concentration-dependent over a physiological range of vitamin concentrations (0.1–100 µM; Figure 1B). Vitamin E-induced increase in the expression level of TTP was also evident in HepG2 cells transiently expressing TTP, as well as in immortalized human hepatocytes (IHH) that endogenously express the protein (Figure 1C). Importantly, two synthetic antioxidants, N-acetyl-cysteine (NAC) and butylated hydroxytoluene (BHT), had no effect on expression levels of the TTP protein under conditions where α-tocopherol caused a marked increase (Figure 1D). Taken together, these observations indicate that vitamin E specifically up-regulates the steady-state expression levels of TTP.
Figure 1. Treatment with vitamin E increases TTP expression levels.
(A). Expression of TTP was induced in HepG2-TetOn-TTP cells with doxycycline. Where indicated, 100µM α-tocopherol was included in the culture media for 48 hours. TTP was visualized in whole cell lysates using anti-HA immunoblotting. (B).. Dose-response of the stabilizing effect of α-tocopherol, conditions as in panel A. (C). Cultured HepG2 cells were transiently transfected with a pCDNA3.1 construct encoding the TTP open reading frame and treated as described above. Forty-eight hours post-transfection, expression of TTP was examined in whole cell lysates by immunoblotting using anti-TTP antibodies 12D7. (D). Immortalized human hepatocytes were treated with α-tocopherol as described above, and expression level of endogenous TTP was examined in whole cell lysates as in panel A. (E) Antioxidant specificity. HePG2-TetOn-TTP cells were cultured as described in panel A. Where indicated, α-tocopherol (0.1 mM), N-acetyl-cysteine (NAC; 1 mM) or butylated hydroxytoluene (BHT, 0.2 mM) were added 48 hrs prior to cell lysis and immunoblotting.
Since α-tocopherol increases the expression levels of ectopically expressed TTP, where an extrinsic promoter drives gene expression, we considered the possibility that the ligand exerts its effect on a post-translational level. Thus, we measured the effect of the vitamin on the turn-over rate of TTP. Cells were incubated with the protein synthesis inhibitor cycloheximide and the levels of TTP were measured at various times. In the absence of α-tocopherol, levels of TTP declined with a half-life (t1/2) of 6 hrs (Figure 2A). Treatment with α-tocopherol slowed the degradation of TTP by 2-fold (t1/2= 12 hrs; Figure 2A). These results indicate that α-tocopherol modulates TTP expression at the level of protein stability, rather than at the level of transcription or translation. In support of this notion, quantitative real-time RT-PCR revealed no α-tocopherol-induced changes in the levels of TTP mRNA in either HepG2-TeTOn-TTP or IHH cells (data not shown).
Figure 2. Alpha-tocopherol protects TTP from proteasomal degradation.
Expression levels of TTP were determined by quantitative densitometric image analysis. (A) HepG2-TetOn-TTP cells were cultured and treated as in Figure 1A. Forty-eight hrs after induction, cycloheximide (CHX; 100 µg/ml) was added for the indicated duration prior to lysis and immunoblotting as in Figure 1. (B–C) The proteasomal inhibitors MG132, Clasto-lactacystin β-lactone or chloroquine were added to 10 µM, 10 µM and 100 µg/ml, respectively for four hrs prior to cell lysis. Shown are averages and standard deviations of three independent experiments.
The specific pathway(s) through which proteins are degraded can be identified with the aid of specific pharmacologic inhibitors. Treatment with MG132 or Clasto-lactacystin β-lactone, inhibitors of the proteasomal degradation pathway (17, 18), markedly attenuated the degradation of TTP (Figure 2B). Inhibition of lysosomal proteolysis with chloroquine, on the other hand, did not affect the rate of TTP degradation (Fig 2C). Taken together, these findings indicate that TTP is subject to degradation by the proteasome, and that binding of α-tocopherol protects the protein from this fate, thereby elevating its steady-state levels.
Proteasomal degradation of proteins involves changes in protein ubiquitination. We therefore examined whether TTP is ubiquitinated, and whether this modification is altered by inclusion of α-tocopherol in the cell culture media. TTP was immuno-precipitated from α-tocopherol- (or control-) treated HepG2-TetOn-TTP cells, and the extent of its modification was assessed by anti-ubiquitin immunoblotting. As shown in Figures 3A, a ubiquitinated protein band was evident at ~40 kDa, in addition to several additional bands at higher molecular weights. These bands represent ubiquitinated forms of TTP since they were visible only when the expression of TTP was induced with doxycycline (compare left- and right-most lanes, Figure 3A). Importantly, treatment of the cells with α-tocopherol all but eliminated TTP ubiquitination (compare middle- and right-most lanes, Figure 3A). These findings reveal that α-tocopherol attenuates the post-translational ubiquitination of TTP. The effect of α-tocopherol is specific to TTP, since treatment with vitamin E did not affect the global protein ubiquitination pattern observed in anti-ubiquitin blots of total cell extracts (Figure 3B). We conclude that the degradation of TTP is regulated by post-translational ubiquitination, and that α-tocopherol increases TTP levels by attenuating the rates of ubiquitination and degradation of the protein.
Figure 3. Alpha-tocopherol attenuates the ubiquitination of TTP.
(A) Expression of TTP was induced for 48 hrs in HepG2-TetOn cells in the absence or presence of 100 µM α-tocopherol. MG132 was added to 10 µM 6 hrs prior to cell lysis. TTP was immunoprecipitated using sepharose-conjugated anti-HA antibodies, and the precipitate was resolved by SDS-PAGE and immunoblotted with anti-HA and anti-ubiquitin antibodies. (B) After precipitation of TTP as in panel A, cell lysates were immunoblotted using the indicated antibodies. Representative of three independent experiments.
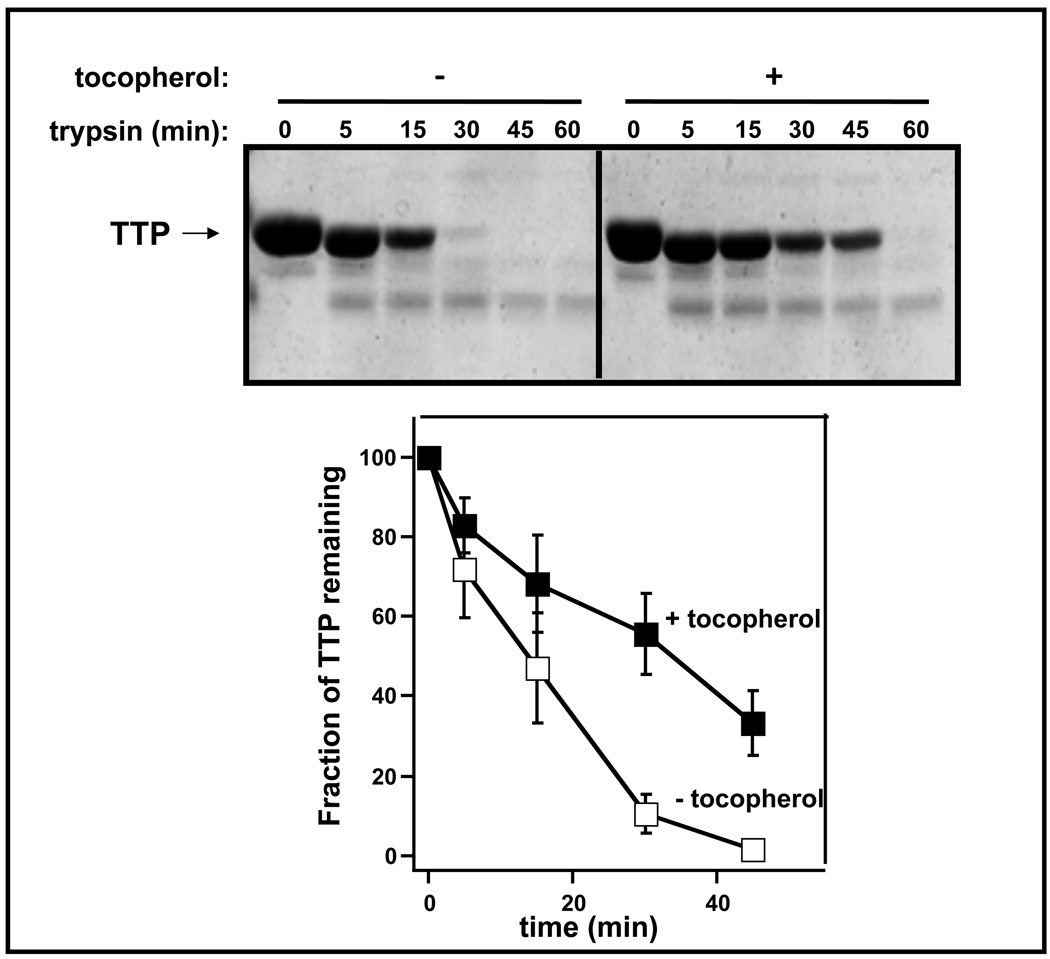
A likely molecular mechanism that underlies the affect of α-tocopherol on the ubiquitination and degradation of TTP involves a ligand-induced conformational change that affects the accessibility of surface residues to components of the ubiquitination machinery. Indeed, comparison of the three-dimensional structures of holo- and apo-TTP reveal that the protein undergoes a significant reorganization upon ligand binding. Specifically, a surface-exposed amphiphatic helix (residues 198–221) functions as a ‘lid’ that closes the binding pocket when it is occupied by α-tocopherol (19, 20). To examine how the ligand-induced conformational changes affect the surface topology of TTP, we monitored the susceptibility of purified TTP to proteolytic degradation by trypsin in vitro. As shown in Figure 4, incubation of purified, recombinant TTP with trypsin lead to efficient degradation of the protein, having a half-life of approximately 15 min. However, when the protein was pre-complexed with α-tocopherol, proteolysis was significantly inhibited. These data indicate that ligand-binding by TTP induces a significant conformational change in the protein, that may sterically hinder accessibility of some surface residues.
Figure 4. Alpha-tocopherol protects TTP from tryptic digestion in vitro.
Twelve µM of purified, recombinant TTP were pre-incubated with 100 µM α-tocopherol (or ethanol control) for 3 hrs prior to the addition of trypsin (7µM). The reaction was incubated at 37°C for the indicated durations prior to SDS-PAGE and Coomassie staining. Bottom: quantification of the time-dependent tryptic proteolysis of TTP. Shown are averages and standard deviations of 4 independent experiments.
To address the physiological consequences of the ligand-induced conformational changes in TTP, we examined whether treatment with α-tocopherol affects the protein’s intracellular localization pattern. Originally, TTP was considered to be a soluble protein, since it was purified from high-speed supernatant of liver homogenate (21). However, recent immunofluorescence microscopy studies have shown that TTP is associated with vesicles of the endocytic pathway, specifically late endosomes and lysosomes (10, 12, 13) These observations raise the possibility that TTP is loosely associated with endocytic vesicles, such that disruption of the cell releases the protein to the cytosol. We therefore used a gentle freeze-thaw lysis protocol, and separated the soluble and organelle-associated fractions by centrifugation. We used this method to determine the localization of TTP in the presence and absence vitamin E treatment, and the rates by which the protein is degraded in the different cellular fractions. As shown in Figure 5, the majority of TTP (>60%) is located at the organelle-associated fraction of the cell. Protein-turnover measurements using cycloheximide revealed that cytosolic TTP was degraded with a half-life of ca. 8 hrs, similar to the rate we observed in total cell lysates (Figures 1,2). Treatment with vitamin E caused a slight (ca. 10%) but consistent decrease in the levels of cytosolic TTP (Figure 5, right panel; compare lanes 1 and 6), suggesting that some TTP translocates away from this compartment. Conversely, treatment with α-tocopherol resulted in significant increase in the levels of organelle-bound TTP (Figure 5, right panel; compare lanes 11 and 16). Vitamin E did not affect the turn-over rate of TTP in the cytosol. The degradation rate of membrane-associated TTP, on the other hand, was significantly retarded in the presence of the vitamin (t1/2 ≫ 16 hrs), such that the levels of TTP in this pool were dramatically increased (Figure 5, right panel). Taken together, these findings show that: 1. TTP is distributed between the cytosol and membranous organelles. 2. Treatment with vitamin E triggers the translocation of a some cytosolic TTP to the membrane-associated pool. 3. Treatment with vitamin E slows down the degradation of organelle-associated TTP, leading to increased levels of the protein in this compartment.
Figure 5. Intracellular distribution of TTP.
Cell culture and treatments were as described in Figure 2. Cells were scraped and lysed by freeze-thawing, and centrifuged to separate the cytosolic (supernatant) and organelle (pellet) fraction. Representative of three independent experiments.
Discussion
Multiple lines of evidence indicate that the hepatic α-tocopherol transfer protein is the main regulator of α-tocopherol status in rodents and humans. First, mutations in the TTPA gene are the only known cause of familial ataxia with vitamin E deficiency, observed in humans with otherwise normal lipid profile (AVED; 6, 22). Second, targeted disruption of the TtpA gene in mice results in vitamin E deficiency, accompanied by neurological symptoms analogous to those displayed by AVED patients (8). Importantly, a linear correlation exists between the steady-state plasma levels of α-tocopherol and TtpA gene osage in the TTP−/−, TTP+/−, and TTP+/+ genotypes (9). Finally, close biochemical examination revealed that of all related lipid-binding proteins from the CRAL_TRIO family, only TTP binds α-tocopherol with high affinity and selectivity (23). It is therefore generally accepted that TTP function in the liver is the key, if not the only, specific biochemical determinant of α-tocopherol levels in humans and rodents (3,4).
TTP is believed to regulate body-wide levels of α-tocopherol by directing the trafficking of the dietary vitamin within the hepatocyte. Newly acquired vitamin E enters hepatic cells by endocytosis of lipoprotein complexes, and α-tocopherol is routed by TTP to export vesicles that deliver it to the plasma membrane (11, 12). Alpha-tocopherol is then secreted from the plasma membrane via an ATP-binding-cassette transporter (12, 24) prior to association with lipoproteins and delivery to peripheral tissues (25–27). Intracellular localization studies provided important clues into the molecular mechanisms of action of TTP. Although hepatic TTP was originally purified from liver cytosol (21), a number of observations suggest that the protein is associated with cellular organelles. Arai and colleagues observed that upon treatment with the lysosomotropic agent chloroquine, TTP’s diffuse distribution pattern changed to a punctate appearance that overlaps that of LBPA, a lipid resident of late endosomes (10). Qian et al. reported that TTP was constitutively associated with EEA1- and LAMP1-positive vesicles, i.e. endosomes and lysosomes (12, 13). These observations suggest that TTP may associate with its ligand at the surface of endocytic vesicles, the vitamin’s ‘port of entry’ into hepatocytes. However, as fluorescence microscopy did not demonstrate a vitamin E-induced change in TTP’s localization pattern, the nature of subsequent steps remained enigmatic.
Here, we employed a gentle detergent-free lysis protocol followed by fractionation and immunoblotting to determine the compartment in which TTP resides. We discovered that >60% of TTP is associated with intracellular organelles. Importantly, treatment of the cells with vitamin E caused a small but significant change in the localization of TTP: ca. 10% of the protein shifted from the cytosolic to the organelle-associated pool. We postulate that TTP binds α-tocopherol on the surface of endocytic vesicles, and dissociates to the cytosol prior to delivering the vitamin to pre-formed transport vesicles that carry it to the plasma membrane. The failure to detect ligand-induced changes in the localization of TTP by immunofluorescence microscopy may have arisen from sensitivity constraints that prevented the detection of small changes.
The critical role that TTP plays in maintaining vitamin E status raises the possibility that antioxidant homeostasis in the face of oxidative stress may be regulated by changes in the protein’s expression levels or activity. This notion prompted a number of animal studies that yielded confusing and sometimes conflicting results. Kim et al reported that increased vitamin E intake lowered the levels of TTP mRNA and protein in rat liver (28). Conversely, Shaw et al. reported that TTP protein levels did not respond to dietary supplementation, but decreased in response to vitamin E depletion (29). When comparing the gene expression profile of vitamin E-deficient versus supplemented rats, Barella et al. noted that the TTP transcript did not change (30). Fechner et al. reported that a supplementation/fasting protocol in vitamin E-depleted rats lead to dramatic increase in TTP mRNA (31). Copp and colleagues reported that the levels of TTP are increased in brain sections of humans afflicted with oxidative stress-related diseases such as AVED, Alzheimer Disease and Down’s Syndrome (32). Recently, Bella et al. observed that TTP protein levels increased in mice supplemented with vitamin E, but did not change in response to smoke-induced oxidative stress (33). To examine the mechanisms that regulate the levels of TTP, we utilized cultured hepatocytes that express TTP endogenously (IHH cells) or ectopically (HepG2 cells). We observed that, in both cell-types, treatment with vitamin E increased the level of TTP protein by 2–3 fold but did not alter the level of TTP mRNA. We found further that vitamin E stabilizes the protein by attenuating the rates at which TTP is ubiquitinated and degraded by the proteasome. As other anti-oxidants did not affect the expression level of TTP, α-tocopherol appears to affect the stability of TTP by inducing a specific conformation rather than through indirect effects on cellular oxidative stress or redox status. This notion is further supported by the findings that α-tocopherol changed TTP’s sensitivity to proteolysis by trypsin in vitro.
The most striking conformational change that accompanies ligand-binding in TTP is a translational movement of residues 198–221 that cover the occupied ligand binding pocket (20). The inter-relationship between ligand-induced conformational changes, ubiquitination pattern, and intracellular localization of TTP remains to be clarified.
Abbreviations
- EDTA
ethylenediaminetetraacetate
- FBS
fetal bovine serum
- HEPES
N-2-Hydroxyethylpiperazine-N'-2-Ethanesulfonic Acid
- PBS
phosphate buffered saline
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide electrophoresis
- TTP
tocopherol transfer protein
Footnotes
Supported by NIH award DK067494 to DM. SM is a recipient of postdoctoral fellowship from the Case Comprehensive Cancer Center (CA59366) and of award 09A107 from the American Institute for Cancer Research.
References
- 1.Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 2.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49:517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 3.Kaempf-Rotzoll DE, Traber MG, Arai H. Vitamin E and transfer proteins. Curr Opin Lipidol. 2003;14:249–254. doi: 10.1097/00041433-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Annu Rev Nutr. 1999;19:343–355. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- 5.Manor D, Morley S. The alpha-tocopherol transfer protein. Vitam Horm. 2007;76:45–65. doi: 10.1016/S0083-6729(07)76003-X. [DOI] [PubMed] [Google Scholar]
- 6.Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998;62:301–310. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hentati A, Deng HX, Hung WY, Nayer M, Ahmed MS, He X, Tim R, Stumpf DA, Siddique T, Ahmed Human alpha-tocopherol transfer protein: gene structure and mutations in familial vitamin E deficiency. Ann Neurol. 1996;39:295–300. doi: 10.1002/ana.410390305. [DOI] [PubMed] [Google Scholar]
- 8.Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H. Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci U S A. 2001;98:15185–15190. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, Suzuki H. Alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J Biol Chem. 2001;276:1669–1672. doi: 10.1074/jbc.C000676200. [DOI] [PubMed] [Google Scholar]
- 10.Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 2003;8:789–800. doi: 10.1046/j.1365-2443.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Arita M, Nomura K, Arai H, Inoue K. alpha-tocopherol transfer protein stimulates the secretion of alpha- tocopherol from a cultured liver cell line through a brefeldin A- insensitive pathway. Proc Natl Acad Sci U S A. 1997;94:12437–12441. doi: 10.1073/pnas.94.23.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. Intracellular trafficking of vitamin E in hepatocytes: Role of tocopherol transfer protein. J Lipid Res. 2005 doi: 10.1194/jlr.M500143-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Qian J, Atkinson J, Manor D. Biochemical Consequences of Heritable Mutations in the alpha-Tocopherol Transfer Protein. Biochemistry. 2006;45:8236–8242. doi: 10.1021/bi060522c. [DOI] [PubMed] [Google Scholar]
- 14.Morley S, Cacchini M, Zhang W, Virgulti A, Noy N, Atkinson J, Manor D. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J Biol Chem. 2008;283:17797–17804. doi: 10.1074/jbc.M800121200. PMC2440614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein is necessary for the maintenance of immortalized human hepatocytes. Virology. 2002;298:53–62. doi: 10.1006/viro.2002.1460. [DOI] [PubMed] [Google Scholar]
- 16.Morley S, Panagabko C, Shineman D, Mani B, Stocker A, Atkinson J, Manor D. Molecular determinants of heritable vitamin E deficiency. Biochemistry. 2004;43:4143–4149. doi: 10.1021/bi0363073. [DOI] [PubMed] [Google Scholar]
- 17.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 19.Min KC, Kovall RA, Hendrickson WA. Crystal structure of human alpha-tocopherol transfer protein bound to its ligand: implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci U S A. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin E retention: structure of human alpha-tocopherol transfer protein. J Mol Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Hagiwara K, Arai H, Inoue K. Purification and characterization of the alpha-tocopherol transfer protein from rat liver. FEBS Lett. 1991;288:41–45. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]
- 22.Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 23.Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Ligand specificity in the CRAL-TRIO protein family. Biochemistry. 2003;42:6467–6474. doi: 10.1021/bi034086v. [DOI] [PubMed] [Google Scholar]
- 24.Oram JF, Vaughan AM, Stocker R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem. 2001;276:39898–39902. doi: 10.1074/jbc.M106984200. [DOI] [PubMed] [Google Scholar]
- 25.Traber MG, Kayden HJ. Vitamin E is delivered to cells via the high affinity receptor for low- density lipoprotein. Am J Clin Nutr. 1984;40:747–751. doi: 10.1093/ajcn/40.4.747. [DOI] [PubMed] [Google Scholar]
- 26.Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med. 2007;28:423–436. doi: 10.1016/j.mam.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Arai H, Arita M, Sato Y, Ogihara T, Inoue K, Mino M, Tamai H. Effect of alpha-tocopherol status on alpha-tocopherol transfer protein expression and its messenger RNA level in rat liver. Free Radic Res. 1998;28:87–92. doi: 10.3109/10715769809097879. [DOI] [PubMed] [Google Scholar]
- 29.Shaw HM, Huang C. Liver alpha-tocopherol transfer protein and its mRNA are differentially altered by dietary vitamin E deficiency and protein insufficiency in rats. J Nutr. 1998;128:2348–2354. doi: 10.1093/jn/128.12.2348. [DOI] [PubMed] [Google Scholar]
- 30.Barella L, Muller PY, Schlachter M, Hunziker W, Stocklin E, Spitzer V, Meier N, de Pascual-Teresa S, Minihane AM, Rimbach G. Identification of hepatic molecular mechanisms of action of alpha-tocopherol using global gene expression profile analysis in rats. Biochim Biophys Acta. 2004;1689:66–74. doi: 10.1016/j.bbadis.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Fechner H, Schlame M, Guthmann F, Stevens PA, Rustow B. alpha- and delta-tocopherol induce expression of hepatic alpha- tocopherol-transfer-protein mRNA. Biochem J. 1998;331:577–581. doi: 10.1042/bj3310577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Copp RP, Wisniewski T, Hentati F, Larnaout A, Ben Hamida M, Kayden HJ. Localization of alpha-tocopherol transfer protein in the brains of patients with ataxia with vitamin E deficiency and other oxidative stress related neurodegenerative disorders. Brain Res. 1999;822:80–87. doi: 10.1016/s0006-8993(99)01090-2. [DOI] [PubMed] [Google Scholar]
- 33.Bella DL, Schock BC, Lim Y, Leonard SW, Berry C, Cross CE, Traber MG. Regulation of the alpha-tocopherol transfer protein in mice: lack of response to dietary vitamin E or oxidative stress. Lipids. 2006;41:105–112. doi: 10.1007/s11745-006-5077-7. [DOI] [PubMed] [Google Scholar]