Abstract
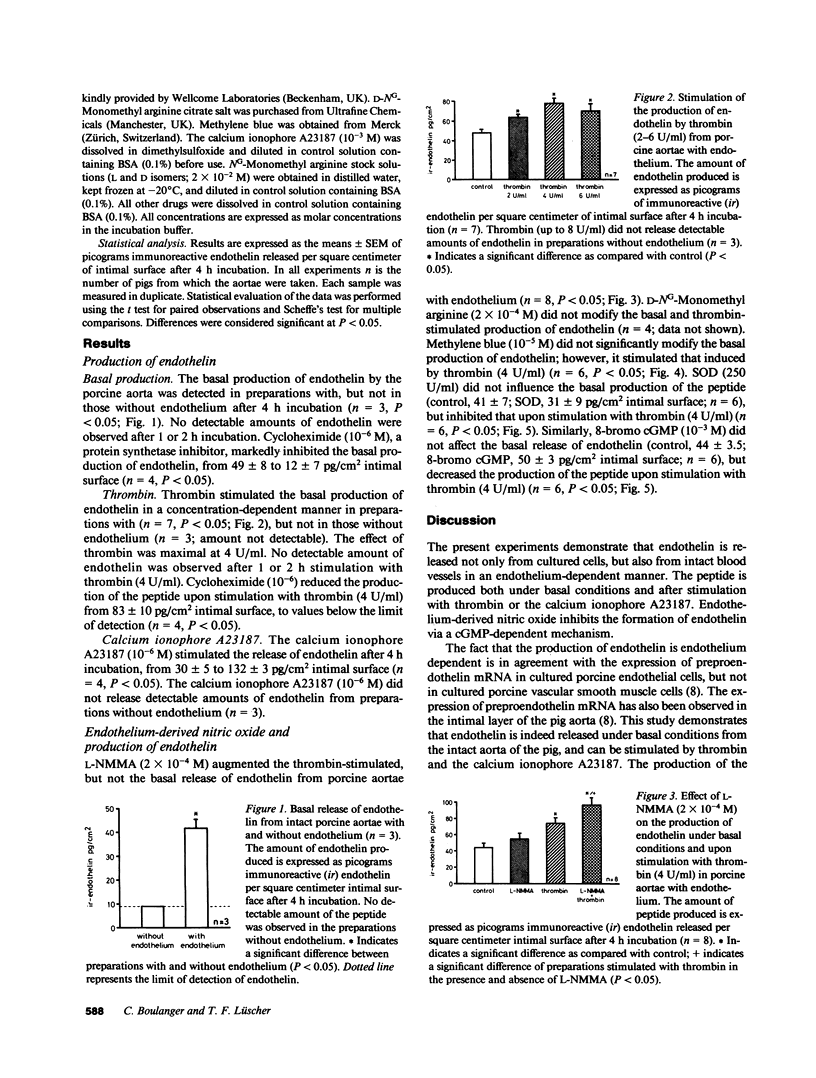
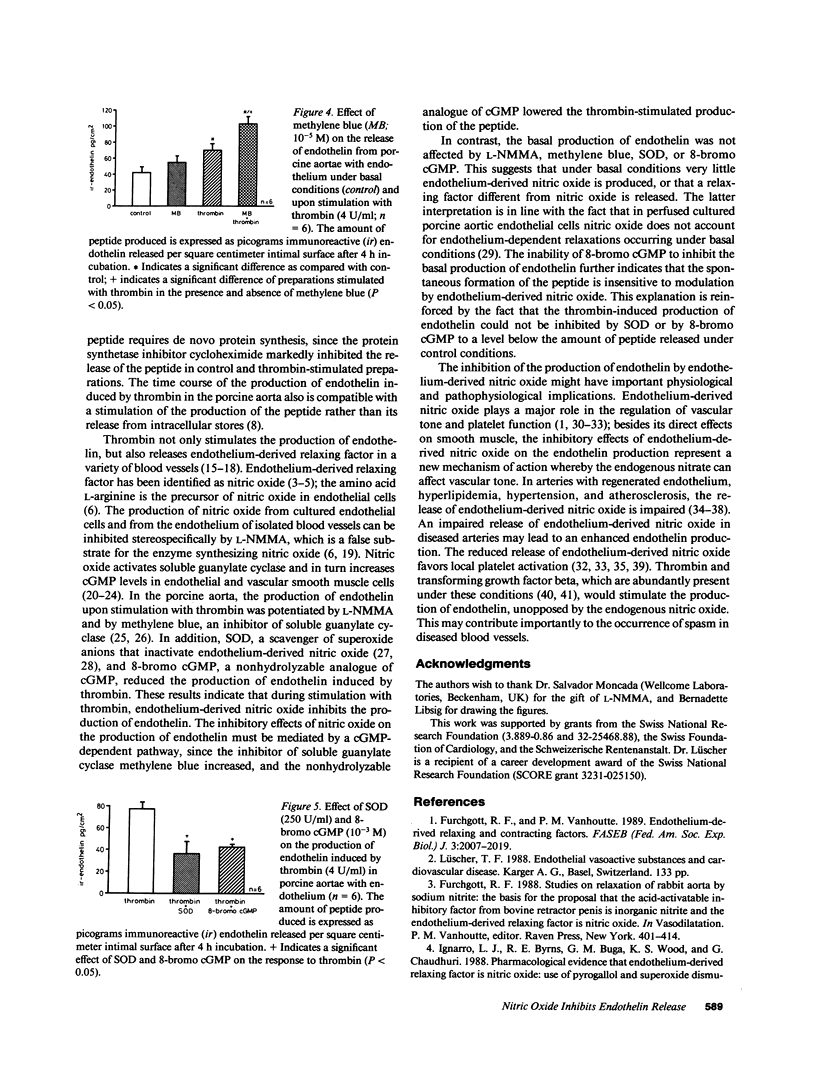
This study was designed to examine whether endothelin is released from the intima of intact arteries, and whether endothelium-derived nitric oxide regulates its production. Endothelin was detected in the incubating medium of unstimulated pig aortae with, but not in those without endothelium. In preparations with endothelium, thrombin (2-6 U/ml) and the calcium ionophore A23187 (10(-6) M) stimulated the release of the peptide. The basal and thrombin-stimulated production of endothelin were prevented by the protein synthetase inhibitor cycloheximide (10(-6) M). The production of endothelin upon stimulation with thrombin (4 U/ml) was potentiated by L-NG-monomethyl arginine and methylene blue and reduced by superoxide dismutase and 8-bromo cyclic guanosine 5'-monophosphate (GMP), while the basal release of the peptide was unaffected. Thus, (a) endothelin is released from the intimal layer of intact blood vessels, both under basal conditions and after stimulation with thrombin and the calcium ionophore A23187, and (b) endothelium-derived nitric oxide released during stimulation with thrombin inhibits the production of the peptide via a cyclic GMP-dependent pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Ohta K., Shichiri M., Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1989 Apr 14;160(1):93–100. doi: 10.1016/0006-291x(89)91625-2. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hickey K. A., Rubanyi G., Paul R. J., Highsmith R. F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985 May;248(5 Pt 1):C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988 Jan;244(1):181–189. [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Lüscher T. F., Diederich D., Siebenmann R., Lehmann K., Stulz P., von Segesser L., Yang Z. H., Turina M., Grädel E., Weber E. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988 Aug 25;319(8):462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F. Endothelium-derived relaxing and contracting factors: potential role in coronary artery disease. Eur Heart J. 1989 Sep;10(9):847–857. doi: 10.1093/oxfordjournals.eurheartj.a059580. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Raij L., Vanhoutte P. M. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension. 1987 Feb;9(2):157–163. doi: 10.1161/01.hyp.9.2.157. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985 Jun;233(3):679–685. [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Mechanisms of adenosine triphosphate-, thrombin-, and trypsin-induced relaxation of rat thoracic aorta. Circ Res. 1984 Oct;55(4):468–479. doi: 10.1161/01.res.55.4.468. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Hendrickson H., Heublein D. M., Burnett J. C., Jr, Vanhoutte P. M. Thrombin enhances the release of endothelin from cultured porcine aortic endothelial cells. Eur J Pharmacol. 1989 Jun 20;165(2-3):333–334. doi: 10.1016/0014-2999(89)90733-4. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Aarhus L. L., Vanhoutte P. M. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ Res. 1987 Aug;61(2):256–270. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Vanhoutte P. M. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res. 1989 May;64(5):900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- Shuman M. A., Majerus P. W. The measurement of thrombin in clotting blood by radioimmunoassay. J Clin Invest. 1976 Nov;58(5):1249–1258. doi: 10.1172/JCI108579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomobe Y., Miyauchi T., Saito A., Yanagisawa M., Kimura S., Goto K., Masaki T. Effects of endothelin on the renal artery from spontaneously hypertensive and Wistar Kyoto rats. Eur J Pharmacol. 1988 Aug 2;152(3):373–374. doi: 10.1016/0014-2999(88)90736-4. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Auch-Schwelk W., Boulanger C., Janssen P. A., Katusic Z. S., Komori K., Miller V. M., Schini V. B., Vidal M. Does endothelin-1 mediate endothelium-dependent contractions during anoxia? J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S124–S142. doi: 10.1097/00005344-198900135-00031. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Houston D. S. Platelets, endothelium, and vasospasm. Circulation. 1985 Oct;72(4):728–734. doi: 10.1161/01.cir.72.4.728. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


