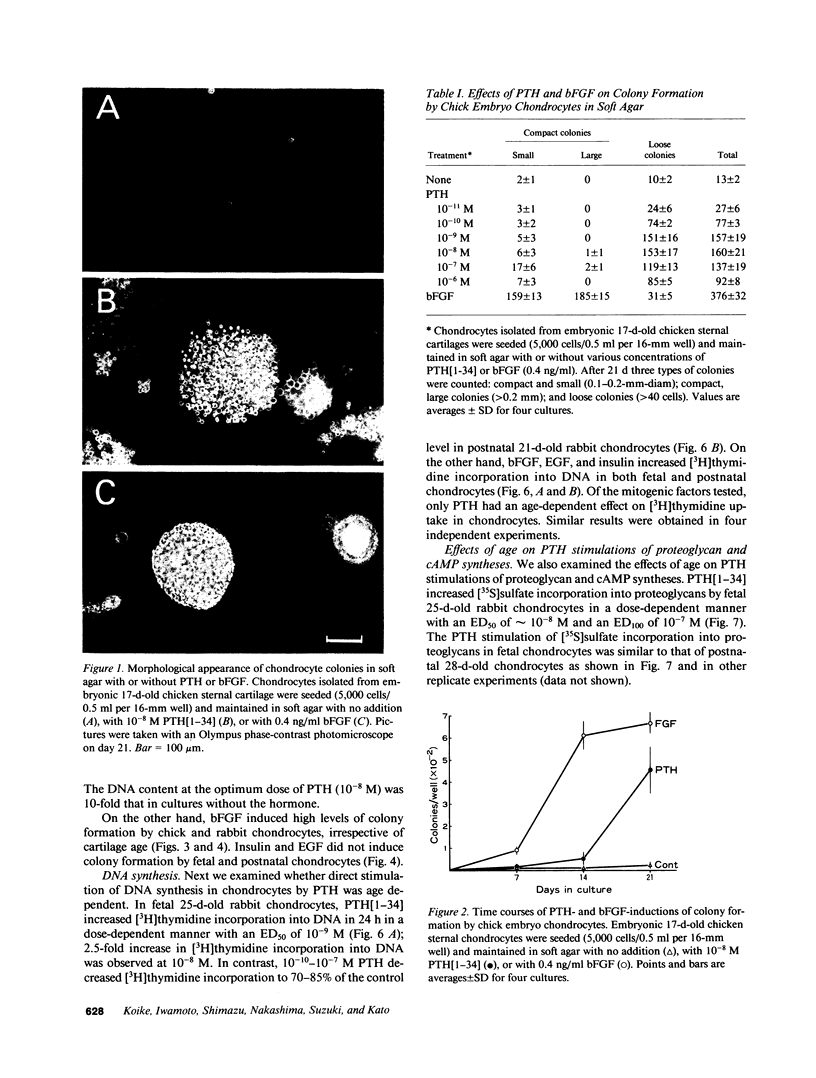
Abstract
The effect of PTH on chondrocyte proliferation as a function of cartilage age was examined. PTH[1-34] induced a 12- to 15-fold increase in the efficiency of colony formation in soft agar by chondrocytes from embryonic 13- to 19-d-old chickens and fetal 25-d-old rabbits with a 10-fold increase in their DNA content. It also caused a 2.5-fold increase in [3H]thymidine incorporation into DNA in fetal 25-d-old rabbit chondrocytes. No mitogenic responses to PTH were observed, however, in postnatal 7- to 21-d-old chick chondrocytes or postnatal 21-d-old rabbit chondrocytes. This age dependency was observed only with PTH: fibroblast growth factor, epidermal growth factor, and insulin stimulated chondrocyte proliferation irrespective of cartilage age. The absence of a mitogenic effect in postnatal chondrocytes was not due to a decrease in number or a reduction in affinity of receptors for PTH. PTH also increased [35S]sulfate incorporation into proteoglycans and the cyclic AMP level in fetal and postnatal chondrocytes, but at 100-fold higher concentrations (10(-8)-10(-7) M) than those (10(-10)-10(-9) M) required for the stimulation of cell division. These results suggest that PTH is a potent mitogen for embryonic chondrocytes, and that its mitogenic effect disappears selectively after birth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bomboy J. D., Jr, Salmon W. D., Jr Effects of cyclic nucleotides on deoxyribonucleic acid synthesis in hypophysectomized rat cartilage: stimulation of thymidine incorporation and potentiation of the action of somatomedin by analogs of adenosine 3',5'-monophosphate or a cyclic nucleotide phosphodiesterase inhibitor. Endocrinology. 1980 Aug;107(2):626–632. doi: 10.1210/endo-107-2-626. [DOI] [PubMed] [Google Scholar]
- Burch W. M., Lebovitz H. E. Parathyroid hormone stimulates growth of embryonic chick pelvic cartilage in vitro. Calcif Tissue Int. 1983 Jul;35(4-5):526–532. doi: 10.1007/BF02405088. [DOI] [PubMed] [Google Scholar]
- Burch W. M., Weir S., Van Wyk J. J. Embryonic chick cartilage produces its own somatomedin-like peptide to stimulate cartilage growth in vitro. Endocrinology. 1986 Sep;119(3):1370–1376. doi: 10.1210/endo-119-3-1370. [DOI] [PubMed] [Google Scholar]
- Canalis E., Centrella M., Burch W., McCarthy T. L. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989 Jan;83(1):60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care A. D., Caple I. W., Singh R., Peddie M. Studies on calcium homeostasis in the fetal Yucatan miniature pig. Lab Anim Sci. 1986 Aug;36(4):389–392. [PubMed] [Google Scholar]
- Carrington J. L., Roberts A. B., Flanders K. C., Roche N. S., Reddi A. H. Accumulation, localization, and compartmentation of transforming growth factor beta during endochondral bone development. J Cell Biol. 1988 Nov;107(5):1969–1975. doi: 10.1083/jcb.107.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drezner M. K., Neelon F. A., Lebovitz H. E. Stimulation of cartilage macromolecule synthesis by adenosine 3',5'-monophosphate. Biochim Biophys Acta. 1976 Apr 2;425(4):521–531. doi: 10.1016/0005-2787(76)90016-2. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Tyler G. A., Krane S. M., Potts J. T., Jr, Rosenblatt M. Photoaffinity labeling of parathyroid hormone receptors: comparison of receptors across species and target tissues and after desensitization to hormone. Biochemistry. 1984 Jan 31;23(3):498–502. doi: 10.1021/bi00298a015. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Yutani Y., Takigawa M., Kato Y., Suzuki F. Differential effects of parathyroid hormone and somatomedin-like growth factors on the sizes of proteoglycan monomers and their synthesis in rabbit costal chondrocytes in culture. Biochim Biophys Acta. 1985 Jun 30;845(3):445–453. doi: 10.1016/0167-4889(85)90210-1. [DOI] [PubMed] [Google Scholar]
- Honma M., Satoh T., Takezawa J., Ui M. An ultrasensitive method for the simultaneous determination of cyclic AMP and cyclic GMP in small-volume samples from blood and tissue. Biochem Med. 1977 Dec;18(3):257–273. doi: 10.1016/0006-2944(77)90060-6. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Sato K., Nakashima K., Fuchihata H., Suzuki F., Kato Y. Regulation of colony formation of differentiated chondrocytes in soft agar by transforming growth factor-beta. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1006–1011. doi: 10.1016/0006-291x(89)92208-0. [DOI] [PubMed] [Google Scholar]
- Kato Y., Gospodarowicz D. Sulfated proteoglycan synthesis by confluent cultures of rabbit costal chondrocytes grown in the presence of fibroblast growth factor. J Cell Biol. 1985 Feb;100(2):477–485. doi: 10.1083/jcb.100.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Iwamoto M., Koike T. Fibroblast growth factor stimulates colony formation of differentiated chondrocytes in soft agar. J Cell Physiol. 1987 Dec;133(3):491–498. doi: 10.1002/jcp.1041330309. [DOI] [PubMed] [Google Scholar]
- Kato Y., Iwamoto M., Koike T., Suzuki F., Takano Y. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9552–9556. doi: 10.1073/pnas.85.24.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Koike T., Iwamoto M., Kinoshita M., Sato K., Hiraki Y., Suzuki F. Effects of limited exposure of rabbit chondrocyte cultures to parathyroid hormone and dibutyryl adenosine 3',5'-monophosphate on cartilage-characteristic proteoglycan synthesis. Endocrinology. 1988 May;122(5):1991–1997. doi: 10.1210/endo-122-5-1991. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nomura Y., Daikuhara Y., Nasu N., Tsuji M., Asada A., Suzuki F. Cartilage-derived factor (CDF) I. Stimulation of proteoglycan synthesis in rat and rabbit costal chondrocytes in culture. Exp Cell Res. 1980 Nov;130(1):73–81. doi: 10.1016/0014-4827(80)90043-9. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nomura Y., Tsuji M., Ohmae H., Kinoshita M., Hamamoto S., Suzuki F. Cartilage-derived factor (CDF). II. Somatomedin-like action on cultured chondrocytes. Exp Cell Res. 1981 Apr;132(2):339–347. doi: 10.1016/0014-4827(81)90109-9. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Iwata S., Endo H. Growth stimulative effect of parathyroid hormone, calcitonin and N6,O2'-dibutyryl adenosine 3';5'-cyclic monophosphoric acid on chick embryonic cartilage cultivated in a chemically defined medium. Endocrinol Jpn. 1980 Jun;27(3):349–356. doi: 10.1507/endocrj1954.27.349. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Kato Y., Tsuji M., Kono T., Hiraki Y., Suzuki F. Prostaglandin stimulation of adenosine 3',5'-monophosphate accumulation in cultured chondrocytes in the presence or absence of parathyroid hormone. Biochim Biophys Acta. 1983 Jun 9;757(3):324–331. doi: 10.1016/0304-4165(83)90058-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewinson D., Silbermann M. Parathyroid hormone stimulates proliferation of chondroprogenitor cells in vitro. Calcif Tissue Int. 1986 Mar;38(3):155–162. doi: 10.1007/BF02556875. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Isgaard J., Lindahl A., Dahlström A., Skottner A., Isaksson O. G. Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science. 1986 Aug 1;233(4763):571–574. doi: 10.1126/science.3523759. [DOI] [PubMed] [Google Scholar]
- Northrop G., Misenhimer H. R., Becker F. O. Failure of parathyroid hormone to cross the nonhuman primate placenta. Am J Obstet Gynecol. 1977 Oct 15;129(4):449–453. doi: 10.1016/0002-9378(77)90593-2. [DOI] [PubMed] [Google Scholar]
- Pitkin R. M., Reynolds W. A., Williams G. A., Kawahara W., Bauman A. F., Hargis G. K. Maternal and fetal parathyroid hormone responsiveness in pregnant primates. J Clin Endocrinol Metab. 1980 Nov;51(5):1044–1047. doi: 10.1210/jcem-51-5-1044. [DOI] [PubMed] [Google Scholar]
- SCOTHORNE R. J. FUNCTIONAL CAPACITY OF FETAL PARATHYROID GLANDS WITH REFERENCE TO THEIR CLINICAL USE AS HOMOGRAFTS. Ann N Y Acad Sci. 1964 Nov 30;120:669–676. doi: 10.1111/j.1749-6632.1964.tb34761.x. [DOI] [PubMed] [Google Scholar]
- Shigeno C., Hiraki Y., Westerberg D. P., Potts J. T., Jr, Segre G. V. Photoaffinity labeling of parathyroid hormone receptors in clonal rat osteosarcoma cells. J Biol Chem. 1988 Mar 15;263(8):3864–3871. [PubMed] [Google Scholar]
- Shimomura Y., Yoneda T., Suzuki F. Osteogenesis by chondrocytes from growth cartilage of rat rib. Calcif Tissue Res. 1975 Dec 22;19(3):179–187. doi: 10.1007/BF02564002. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Yoneda T., Shimomura Y. Calcitonin and parathyroid-hormone stimulation of acid mucopolysaccharide synthesis in cultured chondrocytes isolated from growth cartilage. FEBS Lett. 1976 Nov;70(1):155–158. doi: 10.1016/0014-5793(76)80747-8. [DOI] [PubMed] [Google Scholar]
- Takano T., Takigawa M., Suzuki F. Role of polyamines in expression of the differentiated phenotype of chondrocytes: effect of DL-alpha-hydrazino-delta-aminovaleric acid (DL-HAVA), an inhibitor of ornithine decarboxylase, on chondrocytes treated with parathyroid hormone. J Biochem. 1983 Feb;93(2):591–598. doi: 10.1093/oxfordjournals.jbchem.a134214. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Takano T., Suzuki F. Effects of parathyroid hormone and cyclic AMP analogues on the activity of ornithine decarboxylase and expression of the differentiated phenotype of chondrocytes in culture. J Cell Physiol. 1981 Feb;106(2):259–268. doi: 10.1002/jcp.1041060212. [DOI] [PubMed] [Google Scholar]