Abstract
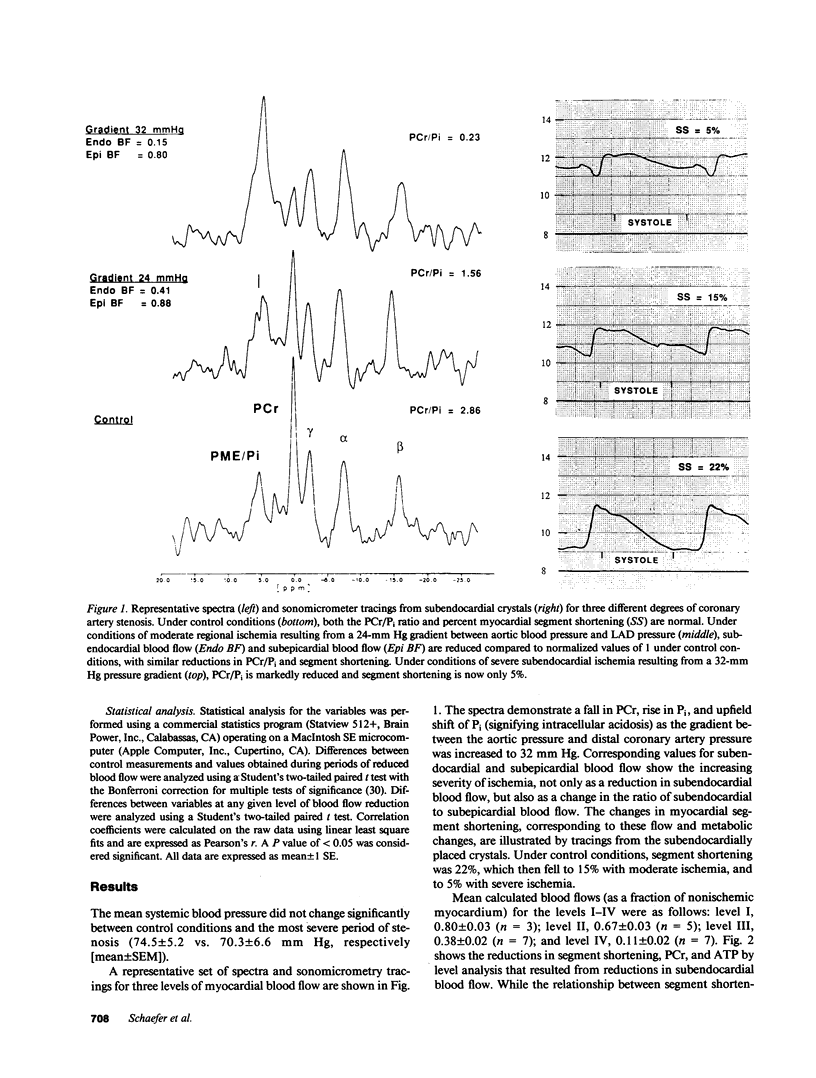
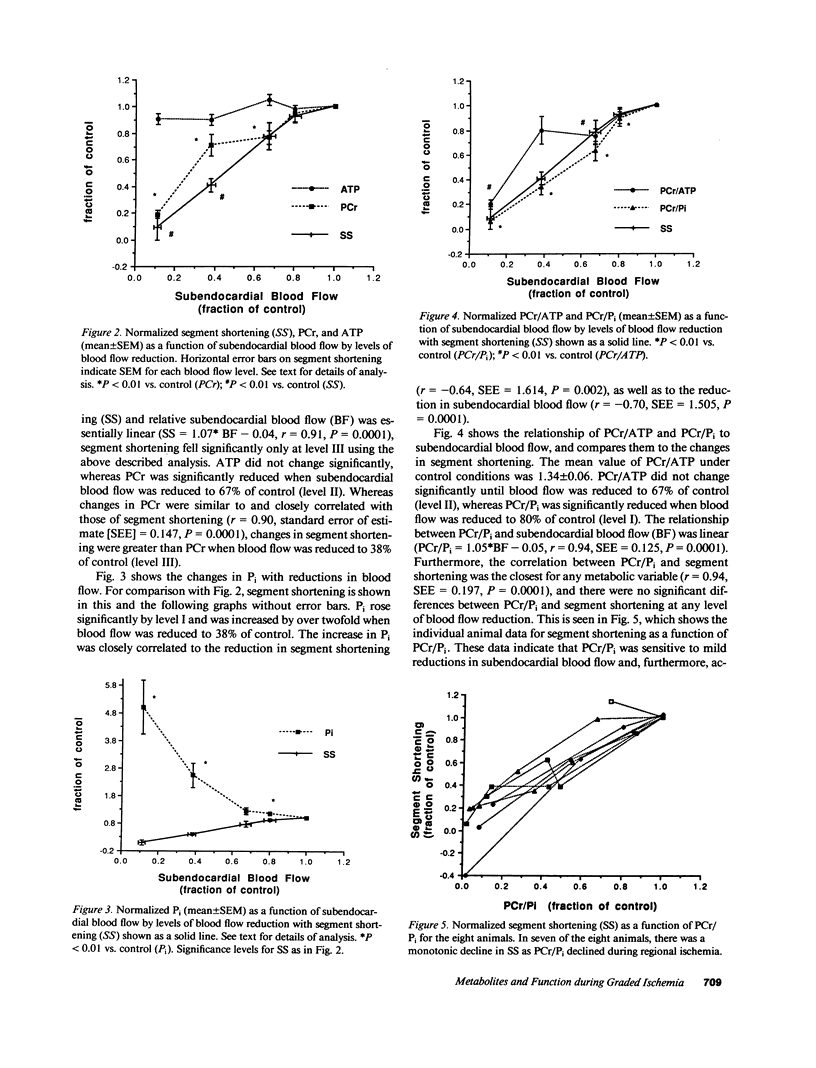
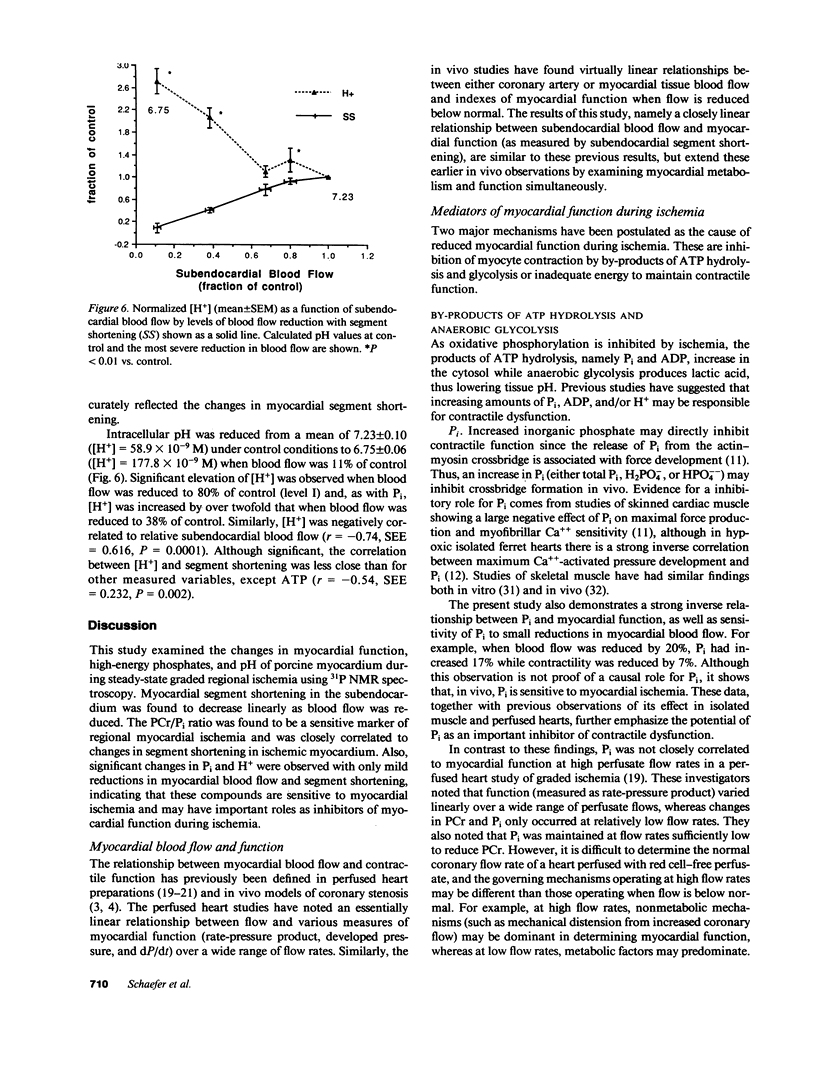
The mechanisms responsible for changes in myocardial contractility during regional ischemia are unknown. Since changes in high-energy phosphates during ischemia are sensitive to reductions in myocardial blood flow, it was hypothesized that myocardial function under steady-state conditions of graded regional ischemia is closely related to changes in myocardial high-energy phosphates. Therefore, phosphorus-31 nuclear magnetic resonance spectroscopy was employed in an in vivo porcine model of graded coronary stenosis. Simultaneous measurements of regional subendocardial blood flow, high-energy phosphates, pH, and myocardial segment shortening were made during various degrees of regional ischemia in which subendocardial blood flow was reduced by 16-94%. During mild reductions in myocardial blood flow (subendocardial blood flow = 83% of nonischemic myocardium), only the ratio of phosphocreatine to inorganic phosphate (PCr/Pi), Pi, and [H+] were significantly changed from control. PCr, ATP, and PCr/ATP were not significantly reduced from control with mild reductions in blood flow. Changes in myocardial segment shortening were most closely associated with changes in PCr/Pi (r = 0.94). Pi and [H+] were negatively correlated with segment shortening (r = -0.64 and -0.58, respectively) and increased over twofold when blood flow was reduced by 62%. Thus, these data demonstrate that PCr/Pi is sensitive to reductions in myocardial blood flow and closely correlates with changes in myocardial function. These data are also consistent with a role for Pi or H+ as inhibitors of myocardial contractility during ischemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittl J. A., Balschi J. A., Ingwall J. S. Effects of norepinephrine infusion on myocardial high-energy phosphate content and turnover in the living rat. J Clin Invest. 1987 Jun;79(6):1852–1859. doi: 10.1172/JCI113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricknell O. L., Daries P. S., Opie L. H. A relationship between adenosine triphosphate, glycolysis and ischaemic contracture in the isolated rat heart. J Mol Cell Cardiol. 1981 Oct;13(10):941–945. doi: 10.1016/0022-2828(81)90292-3. [DOI] [PubMed] [Google Scholar]
- Brooks W. M., Haseler L. J., Clarke K., Willis R. J. Relation between the phosphocreatine to ATP ratio determined by 31P nuclear magnetic resonance spectroscopy and left ventricular function in underperfused guinea-pig heart. J Mol Cell Cardiol. 1986 Feb;18(2):149–155. doi: 10.1016/s0022-2828(86)80467-9. [DOI] [PubMed] [Google Scholar]
- Camacho S. A., Lanzer P., Toy B. J., Gober J., Valenza M., Botvinick E. H., Weiner M. W. In vivo alterations of high-energy phosphates and intracellular pH during reversible ischemia in pigs: a 31P magnetic resonance spectroscopy study. Am Heart J. 1988 Sep;116(3):701–708. doi: 10.1016/0002-8703(88)90327-4. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The homeostasis of calcium in heart cells. J Mol Cell Cardiol. 1985 Mar;17(3):203–212. doi: 10.1016/s0022-2828(85)80003-1. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Kent J., McCully K., Nioka S., Clark B. J., Maris J. M., Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K., O'Connor A. J., Willis R. J. Temporal relation between energy metabolism and myocardial function during ischemia and reperfusion. Am J Physiol. 1987 Aug;253(2 Pt 2):H412–H421. doi: 10.1152/ajpheart.1987.253.2.H412. [DOI] [PubMed] [Google Scholar]
- Clarke K., Willis R. J. Energy metabolism and contractile function in rat heart during graded, isovolumic perfusion using 31P nuclear magnetic resonance spectroscopy. J Mol Cell Cardiol. 1987 Dec;19(12):1153–1160. doi: 10.1016/s0022-2828(87)80525-4. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet J. W., Baartscheer A., Schumacher C. A., Coronel R., ter Welle H. F. The change of the free energy of ATP hydrolysis during global ischemia and anoxia in the rat heart. Its possible role in the regulation of transsarcolemmal sodium and potassium gradients. J Mol Cell Cardiol. 1984 Nov;16(11):1023–1036. doi: 10.1016/s0022-2828(84)80015-2. [DOI] [PubMed] [Google Scholar]
- Gallagher K. P., Matsuzaki M., Koziol J. A., Kemper W. S., Ross J., Jr Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol. 1984 Nov;247(5 Pt 2):H727–H738. doi: 10.1152/ajpheart.1984.247.5.H727. [DOI] [PubMed] [Google Scholar]
- Gallagher K. P., Matsuzaki M., Koziol J. A., Kemper W. S., Ross J., Jr Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol. 1984 Nov;247(5 Pt 2):H727–H738. doi: 10.1152/ajpheart.1984.247.5.H727. [DOI] [PubMed] [Google Scholar]
- Gober J. R., Schaefer S., Camacho S. A., DeGroot M., Obregon R., Botvinick E. H., Weiner M., Massie B. Epicardial and endocardial localized 31P magnetic resonance spectroscopy: evidence for metabolic heterogeneity during regional ischemia. Magn Reson Med. 1990 Feb;13(2):204–215. doi: 10.1002/mrm.1910130204. [DOI] [PubMed] [Google Scholar]
- Guth B. D., Martin J. F., Heusch G., Ross J., Jr Regional myocardial blood flow, function and metabolism using phosphorus-31 nuclear magnetic resonance spectroscopy during ischemia and reperfusion in dogs. J Am Coll Cardiol. 1987 Sep;10(3):673–681. doi: 10.1016/s0735-1097(87)80212-7. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Kammermeier H., Schmidt P., Jüngling E. Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? J Mol Cell Cardiol. 1982 May;14(5):267–277. doi: 10.1016/0022-2828(82)90205-x. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., Allen D. G. Is force production in the myocardium directly dependent upon the free energy change of ATP hydrolysis? J Mol Cell Cardiol. 1986 Sep;18(9):879–884. doi: 10.1016/s0022-2828(86)80001-3. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner R. A., DeBoer L. W., Darsee J. R., Ingwall J. S., Braunwald E. Recovery from prolonged abnormalities of canine myocardium salvaged from ischemic necrosis by coronary reperfusion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7152–7156. doi: 10.1073/pnas.78.11.7152. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Krause S. M., Hess M. L. The effect of short term normothermic global ischemia and acidosis on cardiac myofibrillar Ca2+-Mg2+ ATPase activity. J Mol Cell Cardiol. 1985 May;17(5):523–526. doi: 10.1016/s0022-2828(85)80058-4. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Kübler W., Katz A. M. Mechanism of early "pump" failure of the ischemic heart: possible role of adenosine triphosphate depletion and inorganic phosphate accumulation. Am J Cardiol. 1977 Sep;40(3):467–471. doi: 10.1016/0002-9149(77)90174-6. [DOI] [PubMed] [Google Scholar]
- Marshall R. C. Correlation of contractile dysfunction with oxidative energy production and tissue high energy phosphate stores during partial coronary flow disruption in rabbit heart. J Clin Invest. 1988 Jul;82(1):86–95. doi: 10.1172/JCI113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Boska M. D., Moussavi R. S., Carson P. J., Weiner M. W. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J Clin Invest. 1988 Apr;81(4):1190–1196. doi: 10.1172/JCI113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Ogawa S., Hayashi J., Osuzu F., Hattori S., Takahashi M., Hara K., Tanabe Y., Nakamura Y. Electrophysiologic and myocardial metabolic changes in the acute phase of partial coronary occlusion. Am Heart J. 1983 Oct;106(4 Pt 1):624–630. doi: 10.1016/0002-8703(83)90078-9. [DOI] [PubMed] [Google Scholar]
- Neill W. A., Ingwall J. S. Stabilization of a derangement in adenosine triphosphate metabolism during sustained, partial ischemia in the dog heart. J Am Coll Cardiol. 1986 Oct;8(4):894–900. doi: 10.1016/s0735-1097(86)80432-6. [DOI] [PubMed] [Google Scholar]
- Parmley W. W., Chuck L. Length-dependent changes in myocardial contractile state. Am J Physiol. 1973 May;224(5):1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B., Hill M. L. Total ischemia in dog hearts, in vitro 2. High energy phosphate depletion and associated defects in energy metabolism, cell volume regulation, and sarcolemmal integrity. Circ Res. 1981 Oct;49(4):901–911. doi: 10.1161/01.res.49.4.901. [DOI] [PubMed] [Google Scholar]
- Robitaille P. M., Merkle H., Sublett E., Hendrich K., Lew B., Path G., From A. H., Bache R. J., Garwood M., Uğurbil K. Spectroscopic imaging and spatial localization using adiabatic pulses and applications to detect transmural metabolite distribution in the canine heart. Magn Reson Med. 1989 Apr;10(1):14–37. doi: 10.1002/mrm.1910100103. [DOI] [PubMed] [Google Scholar]
- Schaefer S., Camacho S. A., Gober J., Obregon R. G., DeGroot M. A., Botvinick E. H., Massie B., Weiner M. W. Response of myocardial metabolites to graded regional ischemia: 31P NMR spectroscopy of porcine myocardium in vivo. Circ Res. 1989 May;64(5):968–976. doi: 10.1161/01.res.64.5.968. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Deleeuw G., Rich T., Williamson J. R. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res. 1977 Dec;41(6):849–858. doi: 10.1161/01.res.41.6.849. [DOI] [PubMed] [Google Scholar]
- Stowe D. F., Mathey D. G., Moores W. Y., Glantz S. A., Townsend R. M., Kabra P., Chatterjee K., Parmley W. W., Tyberg J. V. Segment stroke work and metabolism depend on coronary blood flow in the pig. Am J Physiol. 1978 May;234(5):H597–H607. doi: 10.1152/ajpheart.1978.234.5.H597. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T., Ross J., Jr Ca2+ sensitivity change and troponin loss in cardiac natural actomyosin after coronary occlusion. Am J Physiol. 1981 May;240(5):H704–H708. doi: 10.1152/ajpheart.1981.240.5.H704. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Johnson T. A., Buchanan J., Engle C. L., Gettes L. S. Effect of graded coronary flow reduction on ionic, electrical, and mechanical indexes of ischemia in the pig. Circulation. 1987 Nov;76(5):1127–1134. doi: 10.1161/01.cir.76.5.1127. [DOI] [PubMed] [Google Scholar]
- Weiss J., Couper G. S., Hiltbrand B., Shine K. I. Role of acidosis in early contractile dysfunction during ischemia: evidence from pHo measurements. Am J Physiol. 1984 Nov;247(5 Pt 2):H760–H767. doi: 10.1152/ajpheart.1984.247.5.H760. [DOI] [PubMed] [Google Scholar]