Abstract
New neurons are continuously produced in most, if not all, mammals. This Neurogenesis occurs only in discrete regions of the adult brain: the subventricular zone (SVZ) and the subgranular zone (SGZ). In these areas, there are neural stem cells (NSCs), multipotent and selfrenewing, which are regulated by a number of molecules and signaling pathways that control their cell fate choices, survival and proliferation rates. It was believed that growth and morphogenic factors were the unique mediators that controlled NSCs in vivo. Recently, chemokines and cytokines have been identified as important regulators of NSCs functions. Some of the most studied immunological effectors are leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), interferon-gamma (IFN-γ), insulin-like growth factor-1 (IGF-1), tumor necrosis factor alpha (TNF-α), and the chemokines MCP-1 and SDF-1. These substances exert a considerable regulation on proliferation, cell-fate choices, migration and survival of NSCs. Hence, the immune system is emerging as an important regulator of neurogenic niches in the adult brain, but further studies are necessary to fully establish the biological meaning of these neural effects.
Keywords: Interleukin, neural stem cells, subventricular zone, cytokine, chemokine, microglia
Introduction
Tissue-specific stem cells divide to regenerate different cell types for the purpose of tissue maintenance in the adult. For a long time, the brain was considered an exception. Although, proliferating cells were discovered in the mature brain, it was believed that cell proliferation in the brain was limited to glial cells (the supportive cells found around neurons). In the 1960s, this view began to change when new putative microneurons were first described [1]. In the 1980’s neurogenesis and recruitment of new neurons into functional circuits were demonstrated to occur in the telencephalon of adult birds [2, 3]. After that crucial finding, adult neurogenesis was demonstrated in several species such as, mouse, rat, rabbit, cow and primate [4–7]. To date, it is accepted that new neurons are formed and recruited into specific brain circuits probably in all adult vertebrate species, including humans [8].
In the adult brain, active neurogenesis occurs only in discrete regions of the central nervous system (CNS): the subventricular zone (SVZ) and the subgranular zone (SGZ). The source of new neurons in the adult brain is neural stem cells (NSCs). The NSCs, which are multipotent and selfrenewing, are regulated by a number of molecules and signaling pathways. Some of the most studied modulators are epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or FGF-2), platelet derived growth factor (PDGF), Notch, Sonic hedgehog, gp130 and others. Recently, it has been demonstrated that immune system plays a key role in regulating NSCs population through production of chemokines and cytokines. In this review, we describe the main neural niches (the subventricular zone and the subgranular zone), the molecules involved in NSCs regulation and the evidence indicating that several immune mediators control proliferation and cell fate of neural primary progenitors. Since adult NSCs may function as a source of neural precursors for brain repair, elucidating the molecular mechanisms that control their survival, proliferation and fate is a crucial step to design effective procedures to manipulate them.
Subventricular zone (SVZ)
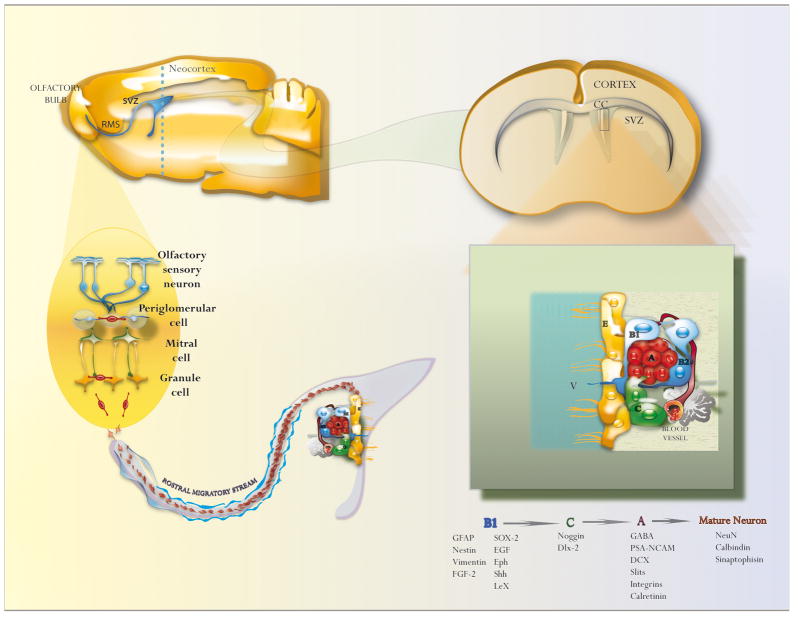
NSCs have been isolated from the SVZ Fig. (1), the lining of the lateral ventricles, and the SGZ of the dentate gyrus Fig. (2), within the hippocampus. The largest of these germinal regions, the SVZ, contains a population of cells that has structural and molecular characteristics of astrocytes, which function as NSCs. Astrocytic NSCs, also called Type-B1 cells, divide to give rise to actively proliferating transit amplifying progenitors (Type-C cells). Type-C cells, in turn, generate neuroblasts (Type-A cells) that migrate anteriorly through the rostral migratory stream (RMS) into the olfactory bulb to become interneurons Fig. (1) [9–12]. Interestingly, Type-B and Type-C cells also generate some oligodendrocytes that migrate and myelinate the neighboring corpus callosum and fimbria fornix [13, 14]. The role of the SVZ-derived interneurons remains unclear but they seem to regulate the olfaction process [15].
Fig. 1. The adult subventricular zone.
Schematic drawing that shows the cellular organization of the adult SVZ, RMS and the olfactory bulb. New neurons born in the SVZ migrate to the olfactory bulb via the RMS. Once SVZ neuroblasts reach the olfactory bulb differentiate into granular and periglomerular GABAergic interneurons. B1: Type-B1 cell; C: Type-C cell; A: Type-A cell; V: Ventricle; CC: Corpus callosum; RMS: Rostral migratory stream; SVZ: Subventricular zone
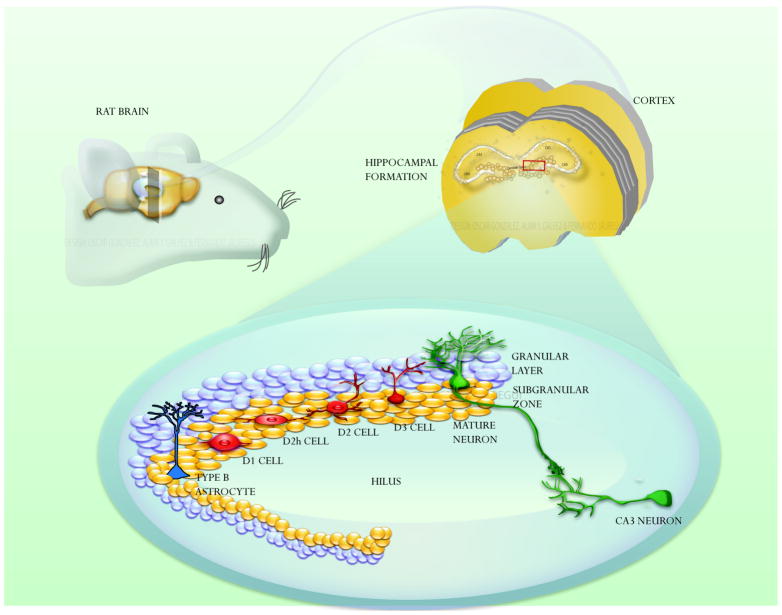
Fig. 2. The subgranular zone.
Schematic drawing that shows the cellular organization of the adult subgranular zone. Radial astrocytes (Type-B cells) give rise to intermediate neuronal progenitors (also known as Type-D cells) that, in turn, differentiate into granular neurons in the dentate gyrus. Mature granular cells synapse to neuronal projections from CA3 and entorhinal cortex. Type-D cells have 4 stages: D1, D2 (radial progenitors), D2h (horizontal progenitors) and D3.
Subgranular zone (SGZ)
The SGZ of the dentate gyrus in the hippocampus is a proliferative region that contains neuronal progenitors that give rise to granular neurons Fig. (2). The primary progenitors in this region are radial astrocytes (Type-B cells) that asymmetrically divide to give rise to Type-D cells [16]. These intermediate progenitors have at least 4 stages of maturation in (D1, D2, D2h and D3) to finally differentiate into granular neurons Fig. (2) [16, 17]. These cells display multipotential characteristic in vitro, but so far, it has not been demonstrated their multipotential properties in vivo. Therefore, some authors called these SGZ precursors as neuronal progenitors instead of NSCs. The function of these newly generated neurons appears to play a fundamental role in memory process, learning and depression.
Control of cell fate and proliferation of NSCs
There is a number of trophic and morphogenic factors that regulate in vivo proliferation of adult NSCs in the neurogenic niches. Table 1 summarizes some of the most studied trophic factors. Members of the fibroblast and epidermal growth factor families are mitogens that expand in vitro and in vivo the adult neural progenitors. Some of these well-studied mediators are: epidermal growth factor (EGF) [14, 18], basic fibroblast growth factor (bFGF or FGF-2) [19], platelet-derived growth factor (PDGF-α) [20], tumor-derived transforming growth factor (TGF-α) [21], brain-derived neurotrophic factor (BDNF) [22], sonic hedgehog (Shh) [23, 24] and others.
Table 1.
Effects of trophic and morphogenic factors on adult NSCs.
| Factor | Proliferation | Predominant cell fate | Reference |
|---|---|---|---|
| EGF | ++++ | Astrocytes and oligodendrocytes | [1] |
| PDGF-α | +++ | Astrocytes and oligodendrocytes | [2] |
| bFGF | +++ | Neurons | [1, 3] |
| TGF-α | +++ | Immature SVZ progenitors | [4, 5] |
| TGF-β | ++ | Astrocytes and neurons | [6, 7] |
| Noggin | ++ | Neurons | [8] |
| GM-CSF | ++ | Neurons | [9] |
| G-CSF | ++ | Neurons | [10] |
| EPO | ++ | Neurons | [11, 12] |
| Shh | ++ | Neurons | [13, 14] |
| VEGF | + | Neurons | [15] |
| BMP | + | Astroglia | [16] |
| BDNF | + | None (in the SVZ). Neurons (in the SGZ) |
[3, 17] |
Cell proliferation: Limited or none (+), mild (++), moderate (+++), and high (++++).
Immunological mediators
During the last decade, increasing evidence indicates that immune system targets neurogenic niches and exerts a considerable effect on proliferation, survival, differentiation and migration of NSCs. Cytokines are immunomodulating polypeptide regulators involved extensively in cellular communication [25]. These substances are present virtually in all nucleated cells, but predominantly in macrophages, endothelium and epithelial cells [26]. The neuropoietic cytokine family includes interleukin-6 (IL-6), interleukin-18 (IL-18), tumor necrosis factor alpha (TNF-α), ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interferon gamma (IFN-γ) and others [25, 27]. Chemokines are small cytokines or proteins, which are categorized into four groups: CXC (or α-chemokines), which promote the migration of neutrophils and lymphocytes; CC chemokines (or β-chemokines), which induce the migration of monocytes, natural killers (NK cells) and dendritic cells; C chemokines (or γ-chemokines) that attract T cell precursors to the thymus; and CX3C chemokines (or δ-chemokines), which serve as chemoattractants and adhesion molecules [26]. Cytokines and chemokines have been shown to alter NSCs self-renewal, progenitor cell division and differentiation that is probably mediated by the Janus kinase-signal transducer and JAK/STAT, an activator of the transcription pathway [25, 27].
Immunological regulation of NSCs
The brain is an immune-privileged organ because the selective permeability of the blood-brain barrier (BBB) only allows certain molecules and cells to enter and leave the cerebral parenchyma. Therefore, under normal physiological conditions, only macrophages, T cells and dendritic cells can go into the nervous system [26, 28, 29]. After damage, an inflammatory process is initiated by the activation of astrocytes and microglia. This event is followed by parenchymal infiltration of macrophages and lymphocytes. These activated and recruited cells release a number of anti- and pro-inflammatory substances, neurotransmitters, chemokines and reactive oxygen species. Then, more inflammatory factors are released, creating a positive feedback loop that results in neural damage and causes both detrimental and positive consequences to neurogenesis [25, 29, 30]. In particular, cytokines/chemokines seem to significantly modify the functions of adult NSCs. Table 2 summarize some of these immunological effectors, but many of them have not been fully characterized.
Table 2.
Effects of chemokines and cytokines on adult NSCs.
| Chemokine / Cytokine | Effect on neural precursor cells | Reference |
|---|---|---|
| IFN- γ | Reduction of proliferation and survival of NSCs. Promotion of differentiation and neurite outgrowth. | [37, 38, 45] |
| IGF-1 | Increasing of neurogenesis in dentate gyrus | [47] |
| IL-4 | Increasing of oligodendrocyte precursors | [48] |
| TNF- α | Decreasing of neurogenesis | [29] |
| Leptin | Inhibition of differentiation of neural progenitors | [27, 35] |
| IL-6 | Differentiation into glutamatergic neurons and oligodendrocytes | [36] |
| MCP-1 | Chemotactic factor of neural precursor | [49, 50] |
| SDF-1 | Chemotactic factor that also promotes survival and proliferation of adult NSCs | [49, 52, 53, 55] |
| CCL2 | Neuronal differentiation of SVZ neural progenitors | [56] |
| LIF and CNTF | Neurogenesis promotions and stem cell self-renewal | [27,31,32,69] |
Acute or chronic exposure to LIF or CNTF differentially affects development and growth of NSCs derived from the adult SVZ [27]. Acute LIF or CNTF exposure stimulates the amplification and self-renewal of NSCs [31–33]. In contrasts, chronic exposure to LIF or CNTF alters the formation of NSC progenies and promotes NSC self-renewal [27]. Intracellular phosphorylation of STAT3 is essential for the effects of LIF in maintaining NSC phenotype [34]. However, leptin, which activates STAT3 after binding to the leptin receptor, inhibits differentiation of multipotent cells [27, 35].
NSCs do not express a functional IL-6R, thus they do not properly respond to IL-6. However, the stimulation of NSCs with the active fusion protein of IL-6 and sIL-6R, also named as Hyper-IL-6 (H-IL-6), induces NSCs to differentiate into glutamate-responsive neurons and oligodendrocytes [36]. The inflammatory cytokine IFN- γ is pro-neurogenic. IFN- γ promotes neural differentiation and neurite outgrowth of murine adult NSCs [37, 38] and the human neuroblastoma cells [39]. Neuronal differentiation induced by IFN-γ appears to be mediated by the c-Jun N-terminal kinase (JNK) pathways [40]. JNK pathway is also required for neural differentiation of embryonal carcinoma cells, embryonic stem cells and PC12 cells [41–44]. However, IFN-γ has shown a dual effect on neurogenesis, because not only stimulates neuronal differentiation [38, 39] and NPC migration, but also inhibits NSCs proliferation and reduces NSCs survival [45]. The manipulation of NSCs with immune mediators may useful to repair brain injuries, as shown by Yang et al. who engineered NSCs to express IL-10, which enhanced their ability to induce immune suppression, remyelination, and neuronal repair [46].
Under pathological conditions, activated microglia produces insulin-like growth factor-1 (IGF-1), which activates the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway, increasing neurogenesis in the SGZ [47]. Microglia activated by IL-4 induces a bias towards oligodendrogenesis whereas the IFN-γ-activated microglia induces a bias towards neurogenesis [48]. In contrast, decreased neurogenesis has been observed by effect of the pro-inflammatory cytokine TNF-α [29]. TNF-α increases the expression of MCP-1, a chemokine that induces NSCs migration mediated through the MCP-1 receptor CCR2 [49, 50]. MCP-1 appears to protect neurons against NMDA-mediated excitotoxicity [51]. SDF-1 chemokyne also induces migration of NPCs and increases survival of NSCS [49, 52, 53], but contrasting reports demonstrated that SDF-1 promotes quiescence [54] or proliferation of neural progenitors [55]. In contrast, the CCL2 chemokine does not affect neural progenitor cell proliferation and cell survival, but promotes neuronal differentiation of SVZ progenitors [56]. Some of these intricate relationships are depicted in Fig. (3).
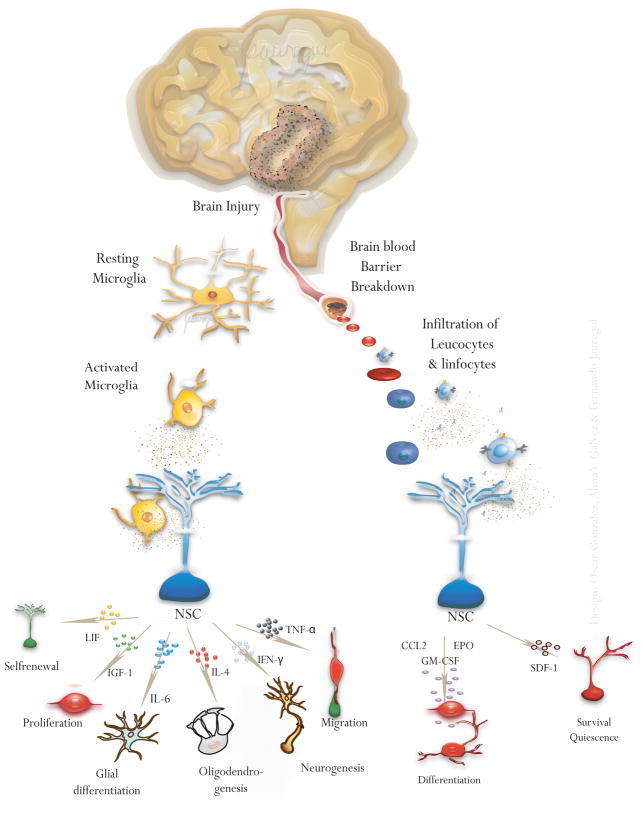
Fig. 3. Effects of cell effectors on NSCs.
After a brain injury, immune cells such as lymphocytes, leucocytes and microglia can induce a number of effects on adult NSCs by releasing a number of cytokines/chemokines in the neurogenic niches.
Hematopoietic growth factors have also been involved in the regulation of adult NSCs, Fig. (3). Granulocyte-macrophage colony stimulating factor (GM-CSF) stimulates neuronal differentiation of adult NSCs [57]. Granulocyte-colony stimulating factor (G-CSF) stimulates neuronal differentiation of NSCs in vitro [58] and enhances neurogenesis and functional recovery. Erythropoietin (EPO) drives neuronal differentiation of NSCs in vitro [59, 60]. Interestingly, EPO-receptor deficient mice display reduced neurogenesis [59, 60].
In summary, immune system is an important regulator of proliferation, migration and survival of NSCs. Yet, as findings in this field are relatively recent, there exist a number of cytokines and chemokines to be investigated. Moreover, signaling pathways involved in all these processes are to be elucidated.
Acknowledgments
O.G-P was supported by CONACyT’s grant (CB-2008-101476) and in part by NIH/NINDS’s grant (1K08NS055851). F.J-H was supported by CONACyT’s posdoctoral fellowship (62022) and A.Y.G-C was supported by CONACyT’s fellowship (214282).
References
- 1.Altman J. Postnatal neurogenesis and the problem of neural plasticity. In: Himwich WA, editor. Developmental neurobiology. C.C. Thomas; Springfield: 1970. pp. 197–237. [Google Scholar]
- 2.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Nottebohm F. Seasonal and species differences in the production of long projection neurons in adult birds. Neuroscience. 1989;15:962. [Google Scholar]
- 4.Galileo DS, et al. Neurons and glia arise from a common progenitor in chicken optic tectum: Demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci USA. 1990;87:458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2.2(4.4):287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 6.Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 7.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22(3):612–3. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro EM, et al. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32(3):1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Perez O, et al. Epidermal Growth Factor Induces the Progeny of Subventricular Zone Type B Cells to Migrate and Differentiate into Oligodendrocytes. Stem Cells. 2009;27(8):2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So K, et al. The olfactory conditioning in the early postnatal period stimulated neural stem/progenitor cells in the subventricular zone and increased neurogenesis in the olfactory bulb of rats. Neuroscience. 2008;151(1):120–8. doi: 10.1016/j.neuroscience.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seri B, et al. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478(4):359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 18.Craig CG, et al. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn HG, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson EL, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinso’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24(41):8924–31. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28(50):13368–83. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machold R, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–50. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 24.Lai K, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–7. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 25.Miller RJ, et al. Chemokine action in the nervous system. J Neurosci. 2008;28(46):11792–5. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonecchi R, et al. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–51. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 27.Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 28.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11(2):125–37. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 29.Whitney NP, et al. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–59. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86(6):1199–208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26(46):12089–99. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima K, et al. LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol. 2007;7:112. doi: 10.1186/1471-213X-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Covey MV, Levison SW. Leukemia inhibitory factor participates in the expansion of neural stem/progenitors after perinatal hypoxia/ischemia. Neuroscience. 2007;148(2):501–9. doi: 10.1016/J.NEUROSCIENCE.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12(9):432–8. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 35.Udagawa J, Nimura M, Otani H. Leptin affects oligodendroglial development in the mouse embryonic cerebral cortex. Neuro Endocrinol Lett. 2006;27(1–2):177–82. [PubMed] [Google Scholar]
- 36.Islam O, et al. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20(1):188–99. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26(9):2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- 38.Wong G, Goldshmit Y, Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187(1):171–7. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Song JH, et al. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem. 2005;280(13):12896–901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ, et al. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochem Res. 2007;32(8):1399–406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- 41.Zentrich E, et al. Collaboration of JNKs and ERKs in nerve growth factor regulation of the neurofilament light chain promoter in PC12 cells. J Biol Chem. 2002;277(6):4110–8. doi: 10.1074/jbc.M107824200. [DOI] [PubMed] [Google Scholar]
- 42.Akiyama S, et al. Activation mechanism of c-Jun amino-terminal kinase in the course of neural differentiation of P19 embryonic carcinoma cells. J Biol Chem. 2004;279(35):36616–20. doi: 10.1074/jbc.M406610200. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, et al. Activation of c-Jun amino-terminal kinase is required for retinoic acid-induced neural differentiation of P19 embryonal carcinoma cells. FEBS Lett. 2001;503(1):91–6. doi: 10.1016/s0014-5793(01)02699-0. [DOI] [PubMed] [Google Scholar]
- 44.Amura CR, et al. Inhibited neurogenesis in JNK1-deficient embryonic stem cells. Mol Cell Biol. 2005;25(24):10791–802. doi: 10.1128/MCB.25.24.10791-10802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Hur T, et al. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24(3):623–31. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119(12):3678–91. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Choi YS, et al. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56(7):791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butovsky O, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Widera D, et al. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83(8):381–7. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- 50.Schwamborn J, et al. Microarray analysis of tumor necrosis factor alpha induced gene expression in U373 human glioblastoma cells. BMC Genomics. 2003;4(1):46. doi: 10.1186/1471-2164-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eugenin EA, et al. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85(5):1299–311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 52.Peng H, et al. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76(1):35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- 53.Molyneaux KA, et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130(18):4279–86. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- 54.Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190(2):216–26. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- 55.Gong X, et al. Stromal cell derived factor-1 acutely promotes neural progenitor cell proliferation in vitro by a mechanism involving the ERK1/2 and PI-3K signal pathways. Cell Biol Int. 2006;30(5):466–71. doi: 10.1016/j.cellbi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Liu XS, et al. Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. J Neurosci Res. 2007;85(10):2120–5. doi: 10.1002/jnr.21359. [DOI] [PubMed] [Google Scholar]
- 57.Kruger C, et al. The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neurosci. 2007;8:88. doi: 10.1186/1471-2202-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider A, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen ZY, et al. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282(35):25875–83. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 60.Shingo T, et al. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733–43. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowenstein DH, Arsenault L. The effects of growth factors on the survival and differentiation of cultured dentate gyrus neurons. J Neurosci. 1996;16(5):1759–69. doi: 10.1523/JNEUROSCI.16-05-01759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fallon J, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97(26):14686–91. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misumi S, et al. Enhanced neurogenesis from neural progenitor cells with G1/S-phase cell cycle arrest is mediated by transforming growth factor beta1. Eur J Neurosci. 2008;28(6):1049–59. doi: 10.1111/j.1460-9568.2008.06420.x. [DOI] [PubMed] [Google Scholar]
- 64.Jordan J, et al. Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. European Journal of Neuroscience. 1997;9(8):1699–709. doi: 10.1111/j.1460-9568.1997.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 65.Bonaguidi MA, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28(37):9194–204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11(3):277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 67.Schanzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–48. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonaguidi MA, et al. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132(24):5503–14. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- 69.Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21(19):7642–53. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]