Abstract
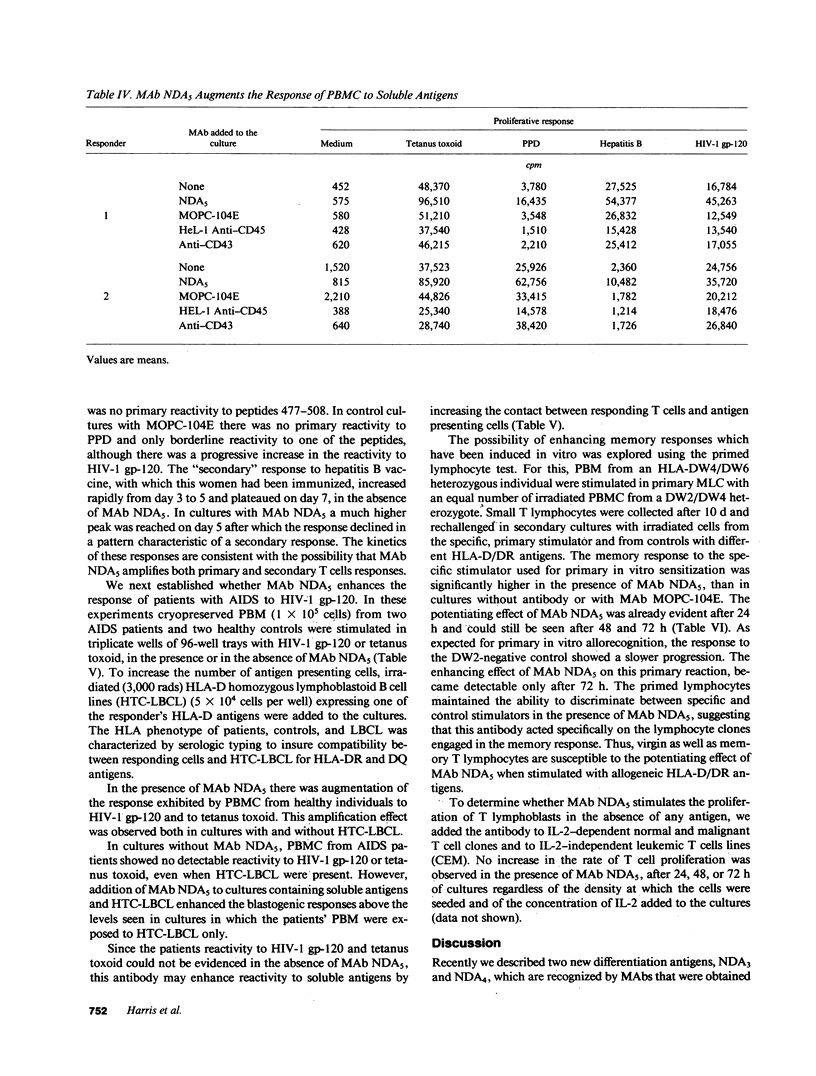
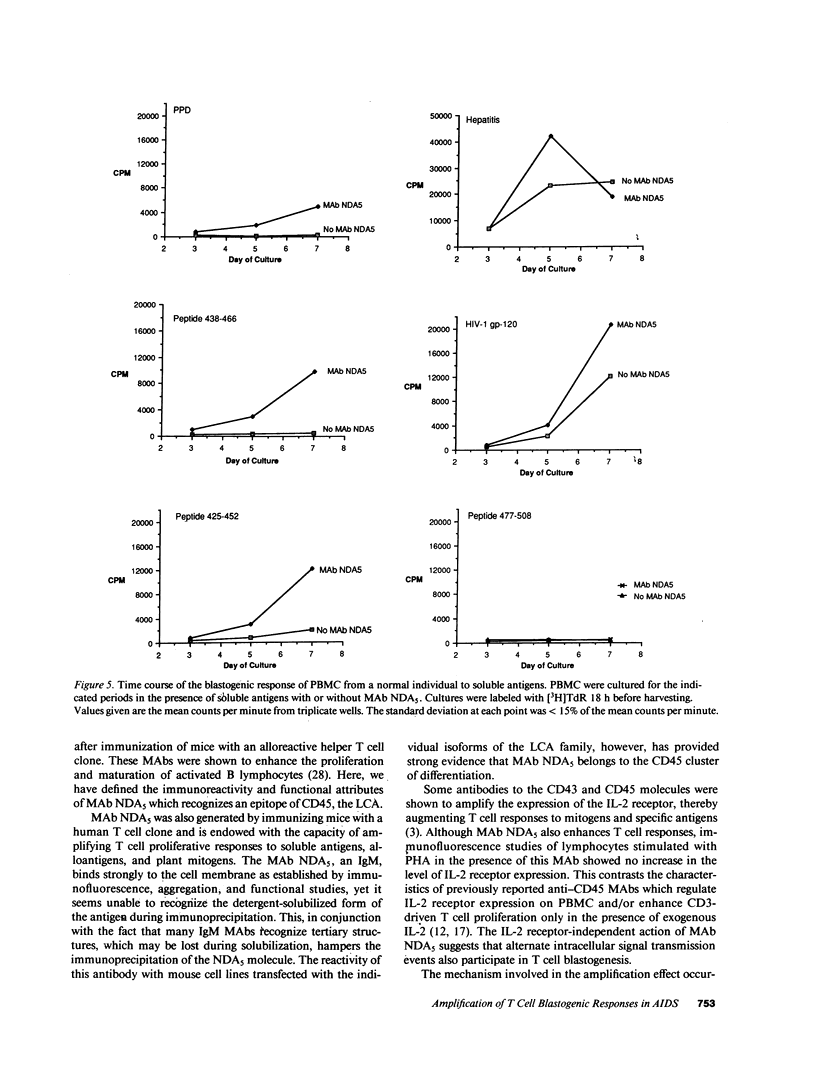
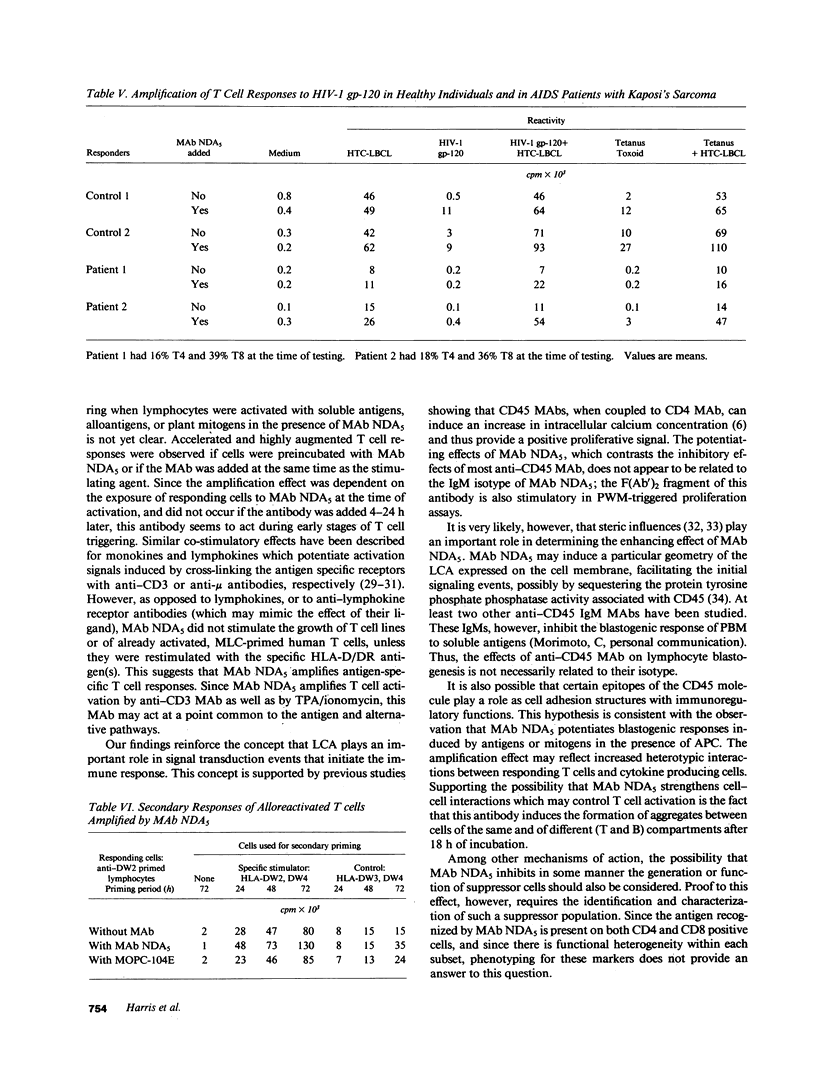
Human immunodeficiency virus (HIV) infection is associated with a profound impairment of T cell function. Hence, enhancement of T cell reactivity to viral and bacterial antigens is important in the treatment of patients with AIDS. To develop tools for amplifying T cell reactivity, we have immunized mice with human helper T cell clones and selected monoclonal antibodies (MAbs) that enhance in vitro blastogenic responses. MAb NDA5, which recognizes the leukocyte common antigen CD45, amplifies human T cell responses to mitogens and soluble antigens including HIV-1 glycoprotein (gp)-120 and peptides derived from the HIV-1 gp-120 sequence. In the presence of MAb NDA5, peripheral blood mononuclear cells (PBMC) from healthy, HIV-1-seronegative individual displayed augmented blastogenic responses to HIV-1 gp-120 and to HIV-1 gp-120 synthetic peptides. In vitro memory responses to various vaccines and to alloantigens were also enhanced in cultures with MAb. Similarly, the response of PBMC from AIDS patients to pokeweed mitogen, HIV-1 gp-120, and tetanus toxoid was enhanced with MAb NDA5. The finding that the in vitro immune response of patients with AIDS can be amplified with MAb NDA5, suggests that the in vivo immune response of immunodeficient individuals can also be enhanced.
Full text
PDF

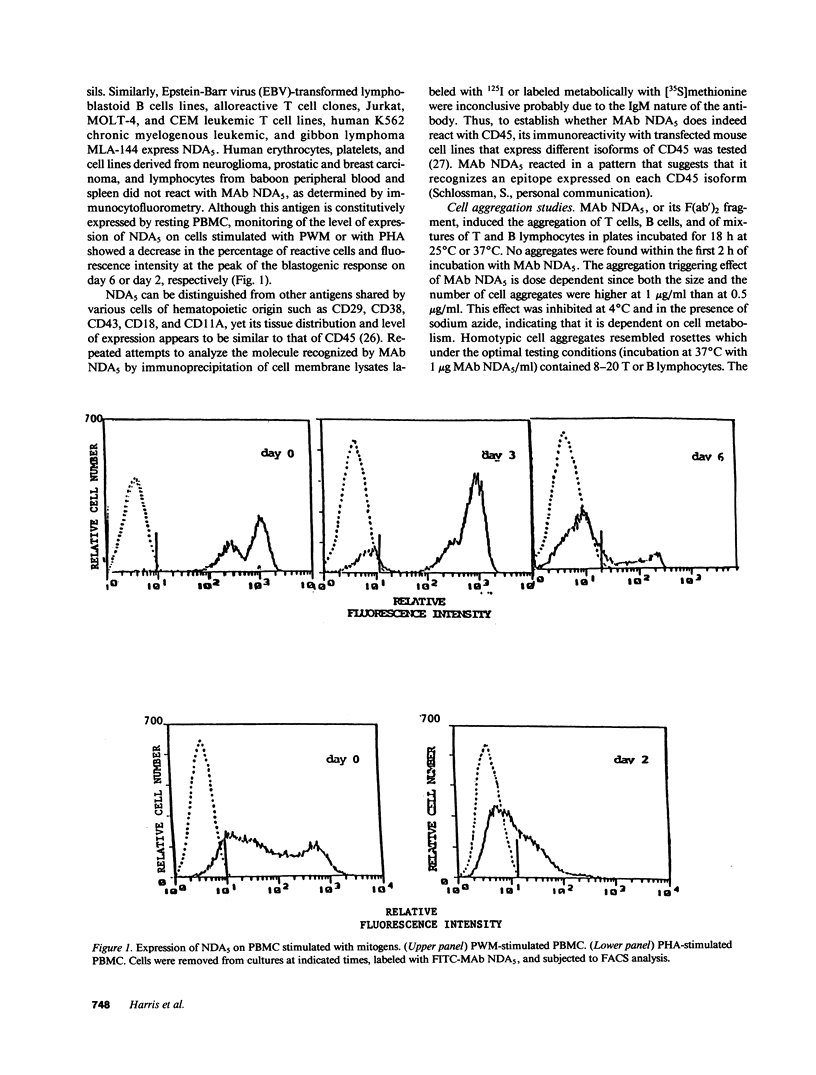
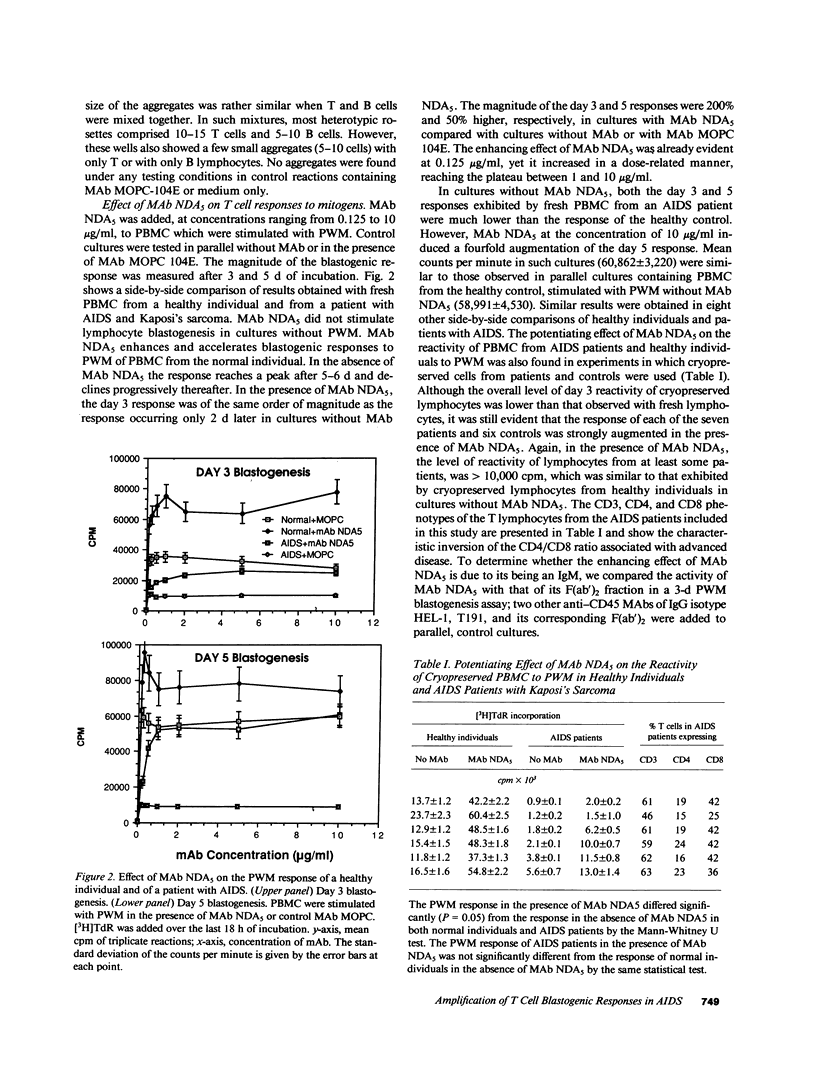
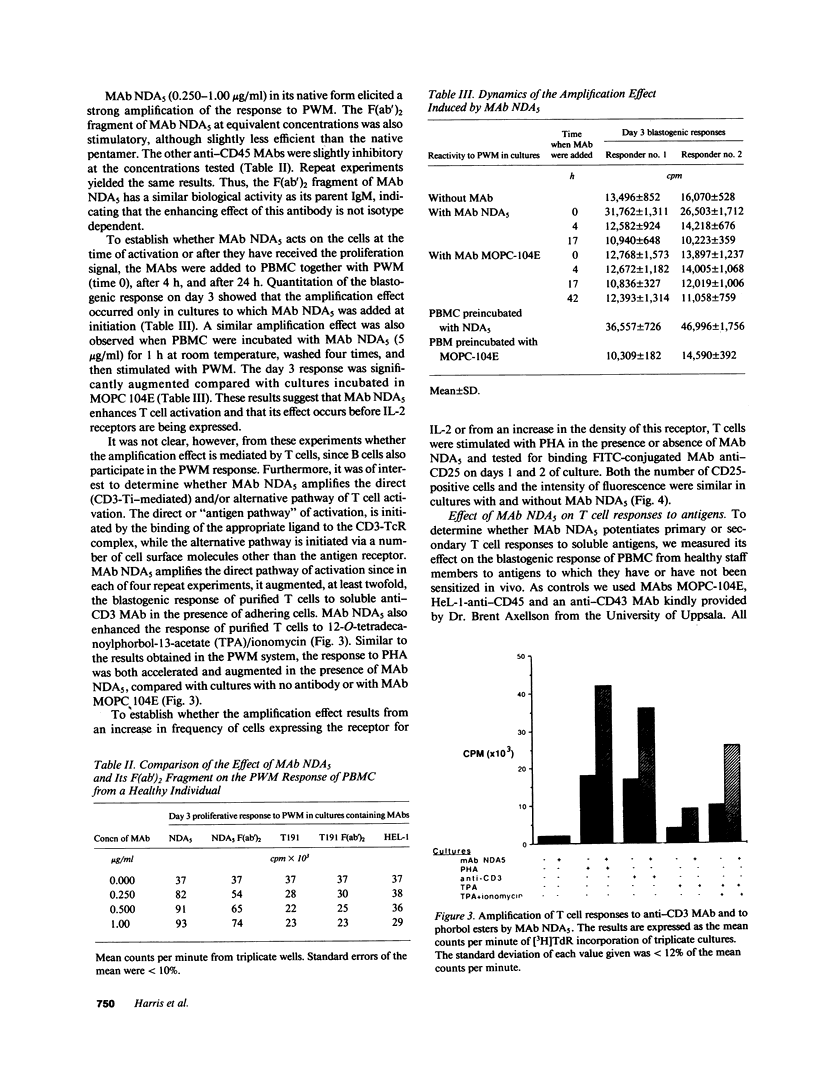
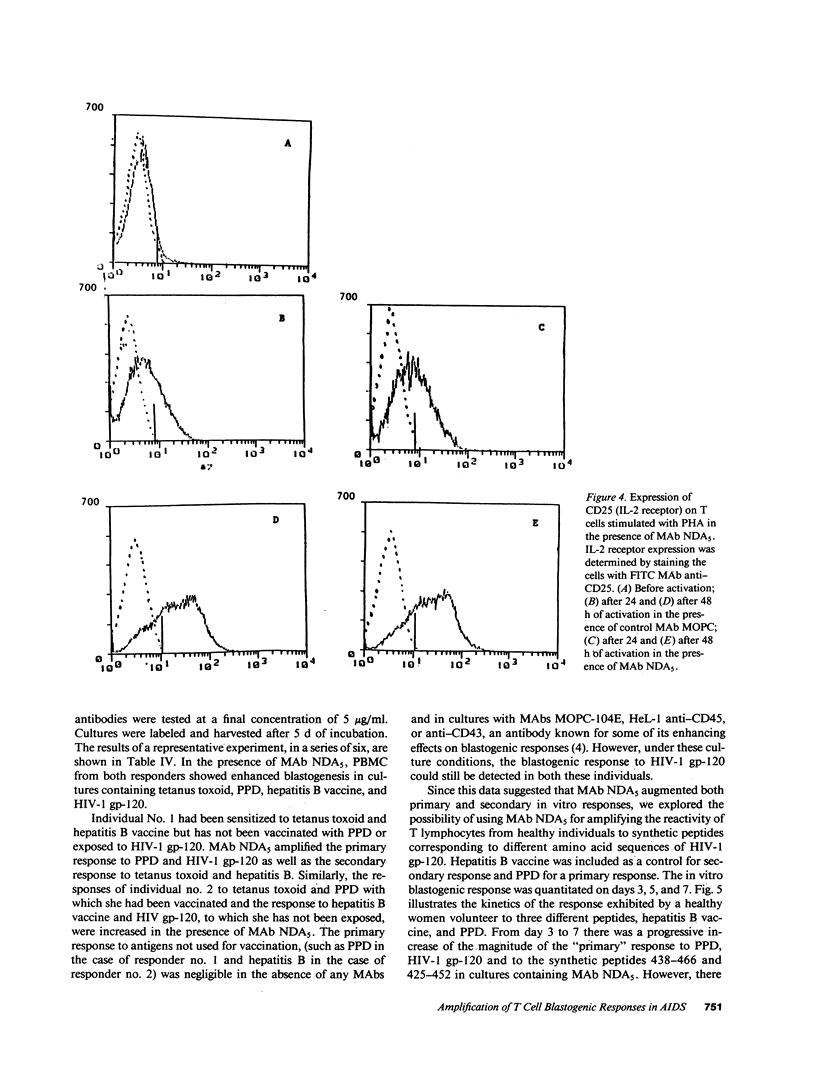


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Ramarli D., Richardson N. E., Chang H. C., Reinherz E. L. Functional and molecular aspects of human T lymphocyte activation via T3-Ti and T11 pathways. Immunol Rev. 1987 Feb;95:5–36. doi: 10.1111/j.1600-065x.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Bierer B. E., Burakoff S. J. T cell adhesion molecules. FASEB J. 1988 Jul;2(10):2584–2590. doi: 10.1096/fasebj.2.10.2838364. [DOI] [PubMed] [Google Scholar]
- Carrera A. C., Rincón M., Sánchez-Madrid F., López-Botet M., de Landaźuri M. O. Triggering of co-mitogenic signals in T cell proliferation by anti-LFA-1 (CD18, CD11a), LFA-3, and CD7 monoclonal antibodies. J Immunol. 1988 Sep 15;141(6):1919–1924. [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haars R., Rohowsky-Kochan C., Reed E., King D. W., Suciu-Foca N. Modulation of T-cell antigen receptor on lymphocyte membrane. Immunogenetics. 1984;20(4):397–405. doi: 10.1007/BF00345614. [DOI] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock K. S., Segal D. M., Hodes R. J. Activation of Lyt-2+ (CD8+) and L3T4+ (CD4+) T cell subsets by anti-receptor antibody. J Immunol. 1989 Apr 1;142(7):2181–2186. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Martin P. J., Spooner C. E., Wofsy D., Tsu T. T., Beatty P. G., Gladstone P. Antibodies to Tp67 and Tp44 augment and sustain proliferative responses of activated T cells. J Immunol. 1985 Oct;135(4):2331–2336. [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. L., Read-Connole E., Arthur L. O., Robey W. G., Wernet P., Schneider E. M., Blattner W. A., Popovic M. HLA-DR is involved in the HIV-1 binding site on cells expressing MHC class II antigens. J Immunol. 1988 Aug 15;141(4):1131–1136. [PubMed] [Google Scholar]
- Martorell J., Vilella R., Borche L., Rojo I., Vives J. A second signal for T cell mitogenesis provided by monoclonal antibodies CD45 (T200). Eur J Immunol. 1987 Oct;17(10):1447–1451. doi: 10.1002/eji.1830171010. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Reichardt L. F. Development and application of an efficient procedure for converting mouse IgM into small, active fragments. J Immunol Methods. 1982;50(3):239–253. doi: 10.1016/0022-1759(82)90162-4. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Rohowsky-Kochan C., Bonagura V., Lewison A., King D. W., Suciu-Foca N. Functional characterization of human alloreactive T cell clones. Hum Immunol. 1984 Dec;11(4):173–182. doi: 10.1016/0198-8859(84)90057-0. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Streuli M., Morimoto C., Schrieber M., Schlossman S. F., Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol. 1988 Dec 1;141(11):3910–3914. [PubMed] [Google Scholar]
- Suciu-Foca N., Cai J. D., Gutiérrea C., Reed E., Morrison S., Pernis B., King D. W. New differentiation antigens associated with the growth and maturation of B lymphocytes. J Immunol. 1988 Jan 15;140(2):395–403. [PubMed] [Google Scholar]
- Suciu-Foca N., Reed E., Rubinstein P., MacKenzie W., Ng A. K., King D. W. A late-differentiation antigen associated with the helper inducer function of human T cells. Nature. 1985 Dec 5;318(6045):465–467. doi: 10.1038/318465a0. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N., Rosochacki S. J., Cai J., Reed E., Rubinstein P., King D. W. Immunological and biochemical characterization of an epitope of the transferrin receptor involved in the production of interferon-gamma and B-cell growth factor. Cell Immunol. 1987 Dec;110(2):265–281. doi: 10.1016/0008-8749(87)90122-5. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Reynolds P. J., Chain A., Ben-Neriah Y., Trowbridge I. S. B-cell variant of mouse T200 (Ly-5): evidence for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5360–5363. doi: 10.1073/pnas.84.15.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]


