Abstract
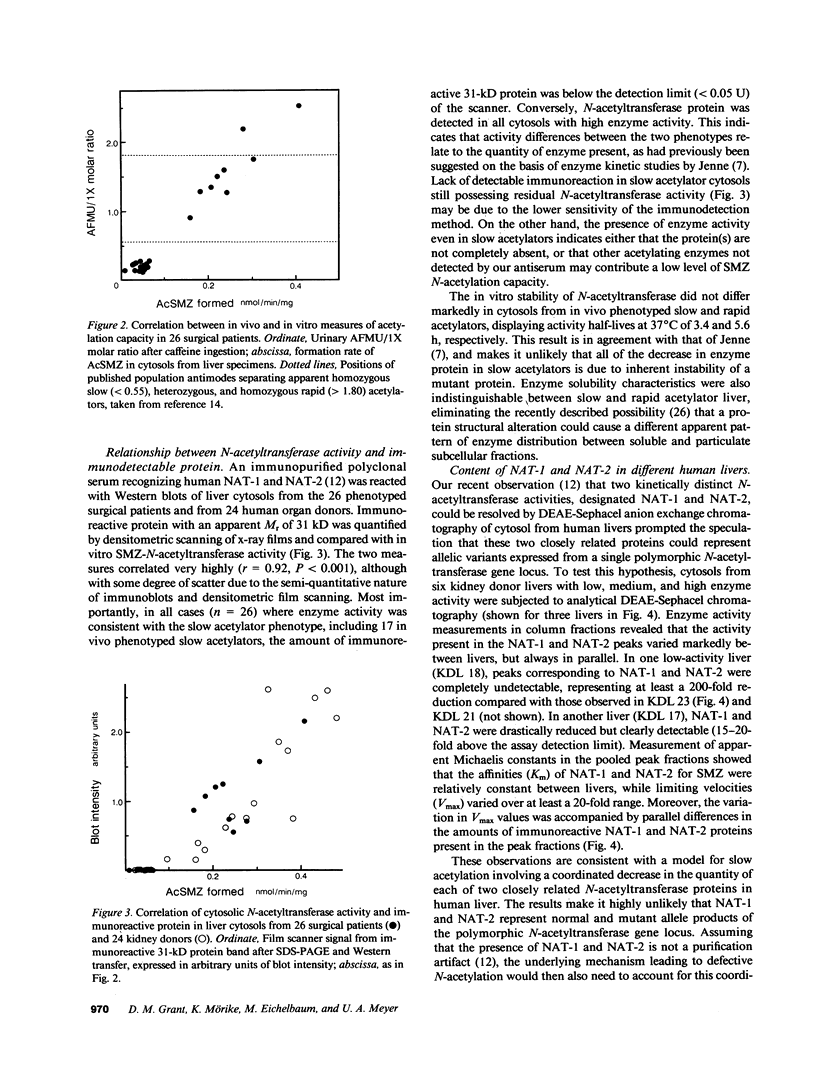
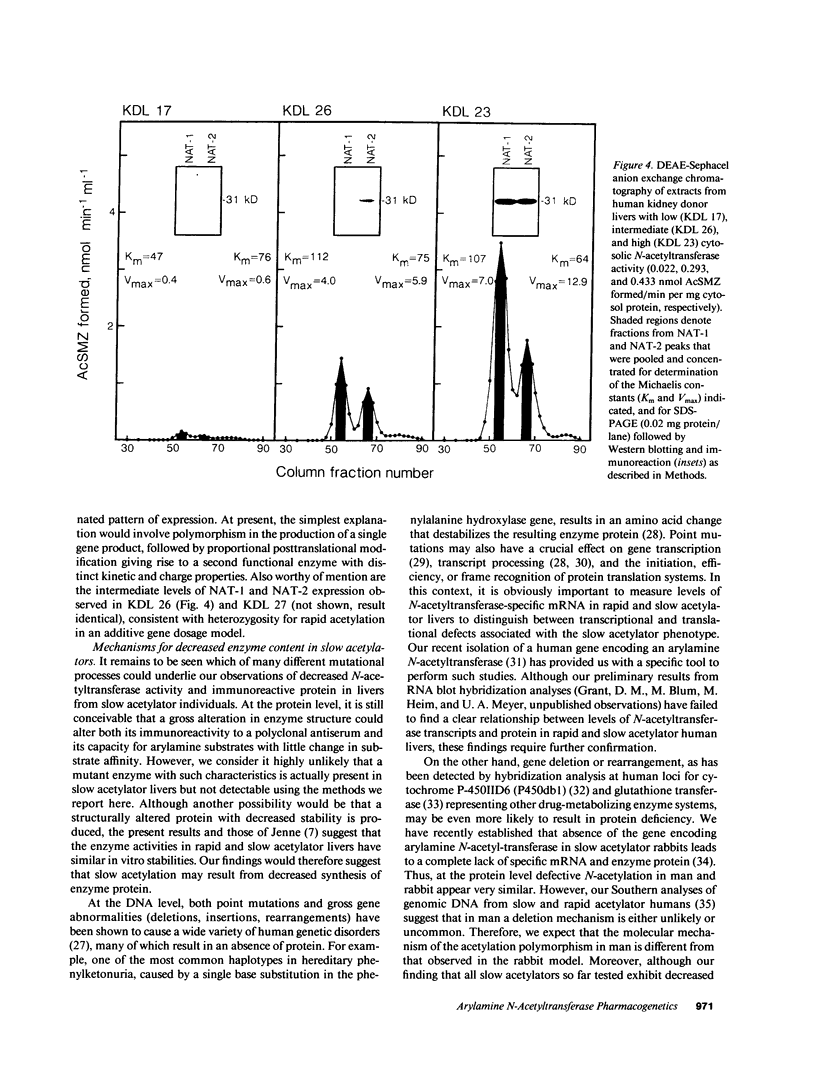
The biochemical basis underlying the genetic polymorphism of drug N-acetylation was investigated using a combination of in vivo and in vitro assays for arylamine N-acetyltransferase (NAT) activity and content in human liver. The acetylator phenotype of 26 surgical patients was determined using caffeine as an innocuous probe drug by measurement of the 5-acetyl-amino-6-formylamino-3-methyluracil to 1-methylxanthine molar ratio in urine. Liver wedge biopsies from these patients and livers from 24 organ donors were then used for measurement of N-acetyltransferase activity with the substrate sulfamethazine and for quantitation of immunoreactive N-acetyl-transferase protein. In vivo (caffeine metabolites in urine) and in vitro (sulfamethazine acetylation) measures of N-acetyl-transferase activity correlated very highly (r = 0.98). Moreover, in all subjects tested, slow acetylation both in vivo and in vitro was associated with a decrease in the quantity of immunodetectable N-acetyltransferase protein in liver cytosol relative to that seen in cytosols from rapid acetylator livers. Two kinetically distinct enzyme activities, designated NAT-1 and NAT-2, were partially purified from low- and high-activity livers and their relationship to acetylator status was determined. Low acetylation capacity was related to decreases in the liver content of both of these immunologically related proteins. The results demonstrate that genetically defective arylamine N-acetylation is due to a parallel decrease in the quantity of two structurally and functionally similar acetylating enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H. H., Klem A. J., Szabo S. M., Weber W. W. New spectrophotometric and radiochemical assays for acetyl-CoA: arylamine N-acetyltransferase applicable to a variety of arylamines. Anal Biochem. 1985 Mar;145(2):367–375. doi: 10.1016/0003-2697(85)90376-8. [DOI] [PubMed] [Google Scholar]
- Andres H. H., Vogel R. S., Tarr G. E., Johnson L., Weber W. W. Purification, physicochemical, and kinetic properties of liver acetyl-CoA:arylamine N-acetyltransferase from rapid acetylator rabbits. Mol Pharmacol. 1987 Apr;31(4):446–456. [PubMed] [Google Scholar]
- Andres H. H., Weber W. W. N-acetylation pharmacogenetics. Michaelis-Menten constants for arylamine drugs as predictors of their N-acetylation rates in vivo. Drug Metab Dispos. 1986 Jul-Aug;14(4):382–385. [PubMed] [Google Scholar]
- Antonarakis S. E. Diagnosis of genetic disorders at the DNA level. N Engl J Med. 1989 Jan 19;320(3):153–163. doi: 10.1056/NEJM198901193200305. [DOI] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. N-acetylation pharmacogenetics: a gene deletion causes absence of arylamine N-acetyltransferase in liver of slow acetylator rabbits. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9554–9557. doi: 10.1073/pnas.86.23.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- EVANS D. A., MANLEY K. A., McKUSICK V. A. Genetic control of isoniazid metabolism in man. Br Med J. 1960 Aug 13;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. A., WHITE T. A. HUMAN ACETYLATION POLYMORPHISM. J Lab Clin Med. 1964 Mar;63:394–403. [PubMed] [Google Scholar]
- Evans D. A. An improved and simplified method of detecting the acetylator phenotype. J Med Genet. 1969 Dec;6(4):405–407. doi: 10.1136/jmg.6.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A. N-acetyltransferase. Pharmacol Ther. 1989;42(2):157–234. doi: 10.1016/0163-7258(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Glowinski I. B., Radtke H. E., Weber W. W. Genetic variation in N-acetylation of carcinogenic arylamines by human and rabbit liver. Mol Pharmacol. 1978 Sep;14(5):940–949. [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Blum M., Demierre A., Meyer U. A. Nucleotide sequence of an intronless gene for a human arylamine N-acetyltransferase related to polymorphic drug acetylation. Nucleic Acids Res. 1989 May 25;17(10):3978–3978. doi: 10.1093/nar/17.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Lottspeich F., Meyer U. A. Evidence for two closely related isozymes of arylamine N-acetyltransferase in human liver. FEBS Lett. 1989 Feb 13;244(1):203–207. doi: 10.1016/0014-5793(89)81193-7. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984 Apr;17(4):459–464. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. Polymorphic N-acetylation of a caffeine metabolite. Clin Pharmacol Ther. 1983 Mar;33(3):355–359. doi: 10.1038/clpt.1983.45. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983 May;33(5):591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hardy B. G., Lemieux C., Walker S. E., Bartle W. R. Interindividual and intraindividual variability in acetylation: characterization with caffeine. Clin Pharmacol Ther. 1988 Aug;44(2):152–157. doi: 10.1038/clpt.1988.130. [DOI] [PubMed] [Google Scholar]
- Jennne J. W. Partial purification and properties of the isoniazid transacetylase in human liver. Its relationship to the acetylation of p-aminosalicylic acid. J Clin Invest. 1965 Dec;44(12):1992–2002. doi: 10.1172/JCI105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Igarashi M., Tanizawa Y., Yamada M., Kishi F., Kajii T., Fujii H., Miwa S., Sakurai M., Nakazawa A. Human adenylate kinase deficiency associated with hemolytic anemia. A single base substitution affecting solubility and catalytic activity of the cytosolic adenylate kinase. J Biol Chem. 1989 Jun 15;264(17):10148–10155. [PubMed] [Google Scholar]
- Meier P. J., Mueller H. K., Dick B., Meyer U. A. Hepatic monooxygenase activities in subjects with a genetic defect in drug oxidation. Gastroenterology. 1983 Sep;85(3):682–692. [PubMed] [Google Scholar]
- Orkin S. H., Sexton J. P., Cheng T. C., Goff S. C., Giardina P. J., Lee J. I., Kazazian H. H., Jr ATA box transcription mutation in beta-thalassemia. Nucleic Acids Res. 1983 Jul 25;11(14):4727–4734. doi: 10.1093/nar/11.14.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E., Radtke H. E., Weber W. W. Immunochemical studies of rabbit N-acetyltransferases. Mol Pharmacol. 1980 May;17(3):367–373. [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. K., Grant D. M., Kalow W. Isolation and identification of 5-acetylamino-6-formylamino-3-methyluracil as a major metabolite of caffeine in man. Drug Metab Dispos. 1983 May-Jun;11(3):218–220. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W. W. The molecular basis of hereditary acetylation polymorphisms. Drug Metab Dispos. 1986 Jul-Aug;14(4):377–381. [PubMed] [Google Scholar]
- Woo S. L. Molecular basis and population genetics of phenylketonuria. Biochemistry. 1989 Jan 10;28(1):1–7. doi: 10.1021/bi00427a001. [DOI] [PubMed] [Google Scholar]
- Zanger U. M., Vilbois F., Hardwick J. P., Meyer U. A. Absence of hepatic cytochrome P450bufI causes genetically deficient debrisoquine oxidation in man. Biochemistry. 1988 Jul 26;27(15):5447–5454. doi: 10.1021/bi00415a010. [DOI] [PubMed] [Google Scholar]



